Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use.
Indication For Use
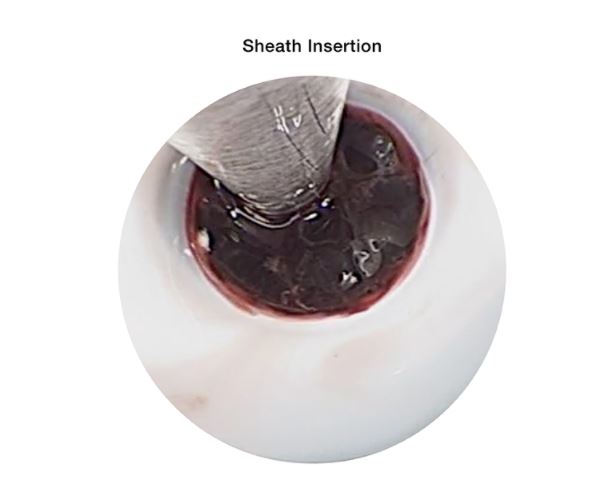
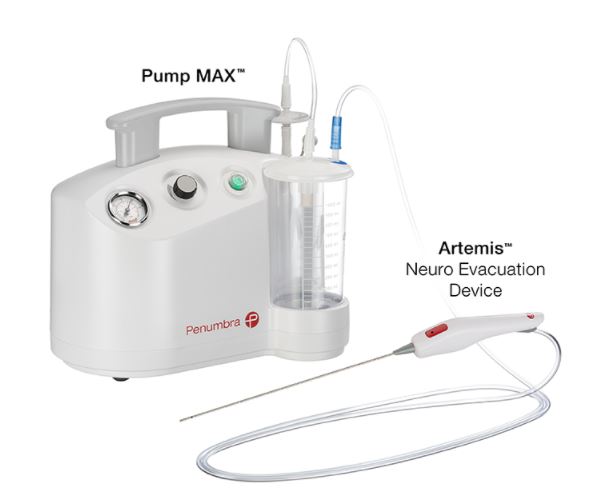
The Artemis Neuro Evacuation Device is used for the controlled aspiration of tissue and/or fluid during surgery of the Ventricular System or Cerebrum in conjunction with a Penumbra Aspiration Pump.
Penumbra Aspiration Pump
The Penumbra Aspiration Pump is indicated as a vacuum source for the Penumbra Aspiration Systems.
Contraindications
• The Artemis Neuro Evacuation Device is not recommended during surgery of the brainstem, cerebellum, epidural or subdural spaces.
• Do not use fibrinolytic therapy during the procedure.
• Do not use the Artemis Neuro Evacuation Device with a non-Penumbra recommended Aspiration Pump. The safety and effectiveness of its use with a non-Penumbra recommended Aspiration Pump has not been established and can lead to patient injury or death.
Warnings
• The Artemis Neuro Evacuation Device should only be used by physicians who have received appropriate training to perform image-guided neurosurgical procedures.
Precautions
• The Artemis Neuro Evacuation Device is intended for single use only. Do not resterilize or reuse. Resterilization or reuse could lead to infection or ineffective removal of tissue and/or fluid.
• Do not use kinked or damaged devices. Do not use open or damaged packages. Return damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use the Artemis Neuro Evacuation Device in conjunction with intraprocedural image-guidance.
• Do not use in an oxygen rich environment.
• Do not advance or use the Artemis Neuro Evacuation Device against resistance without careful visual assessment of the cause. If the cause cannot be determined, withdraw the device. Unrestrained torqueing or forced insertion of the device against resistance may result in damage to the device, which may lead to tissue damage and/or device breakage.
Potential Adverse Events
Possible complications include, but are not limited to, the following:
hematoma expansion, fever, headaches, vomiting, hyperglycemia, edema, re-bleeding, death, bleeding, increased blood pressure, infections, seizures, intraventricular hemorrhage, hydrocephalus, thromboembolic events, decreased consciousness, craniotomy, unintended removal of tissue leading to neurological and/or sensory deficit.
Indication For Use
The Penumbra Pump MAX is indicated as a vacuum source for the Penumbra Aspiration Systems.
Contraindications
There are no contraindications.
Warnings/Precautions
• The canister/tubing is intended for single use only. Do not reuse. Reuse may result in canister cracking or tubing blockages, which may result in the inability to aspirate.
• Do not block bottom or back air vents. Unit may overheat and shut off or fail to restart if run for extended periods of time without airflow.
• To avoid risk of electric shock, this equipment must only be connected to a supply mains with protective earth.
• Do not position the pump so that it is difficult to operate the power cord disconnection device.
• Remove and service the pump if liquids or solids have been drawn into the vacuum pump.
• Do not use in the presence of a flammable anaesthetic mixture with air or nitrous oxide.
• Do not use in oxygen rich environment.
• To prevent fire or shock hazard, use replacement fuses of equal size and rating.
• To prevent fire or shock hazard, use a replacement power cord of equal rating.
• Do not re-infuse blood or fluid from the canister back into patient.
• Do not use petroleum base compounds, acids, caustics, or chlorinated solvents to clean or lubricate any parts. It will reduce the service life of the pump. Use only water-base solvents for cleaning.
• Federal (USA) law restricts this device to sale by or on the order of a physician.
• No modification of this equipment is allowed.