Manufacturer > Angiodynamics > Devices > 400 Micron Perforator & Accessory Vein Ablation Kit (PVAK)
400 Micron Perforator & Accessory Vein Ablation Kit (PVAK)
Device-Type
Ablation System
Manufacturer
Angiodynamics
AngioDynamics’ VenaCure EVLT endovenous laser vein treatment offers patients a minimally-invasive choice for treating the source of their varicose veins and provides them with a potentially quicker recovery and return to normal daily routines, as compared to surgical stripping.
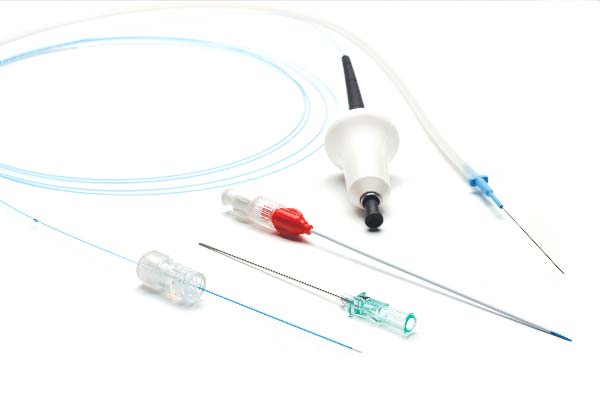
The 400 Micron Perforator and Accessory Vein Ablation Kit (PVAK) allows for simple access and accurate positioning.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×