Manufacturer > Cook Medical > Devices > Advance® ATB PTA Dilatation Catheter
Advance® ATB PTA Dilatation Catheter
Device-Type
PTA Ballons
Targated Speciality
Cardiovascular
Manufacturer
Cook Medical
Used in Procedure
Any Procedure
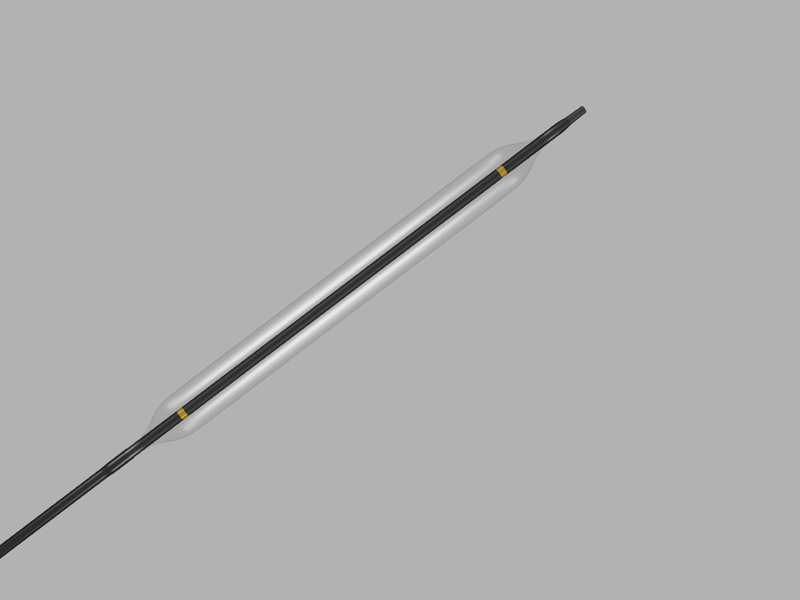
The ATB ADVANCE PTA Dilatation Catheter is a double-lumen catheter with a balloon near its distal tip. The catheter consists of two independent lumens, which are labeled “DISTAL” and “BALLOON.” The distal lumen extends the length of the catheter and is used for placement of wire guides. The balloon lumen is used to expand the balloon. Inscribed on the tip of the manifold are the balloon diameter (mm) and the balloon length (cm).
The balloon is manufactured from an extra-thinwall, high-strength, minimally- compliant material. Particular care should be taken in handling the balloon to prevent damage. It will inflate to the indicated size parameters when utilizing proper pressure recommendations. Adhere to balloon inflation pressure parameters indicated in. Refer to label for further information. Use of a pressure gauge is recommended to monitor inflation pressures.
Gold, radiopaque markers are positioned on the shaft within the balloon to enable visualization of the catheter/balloon under fluoroscopy. The catheter is compatible with .035 inch (0.89 mm) wire guides.
Safety informations
Potential adverse events
Device Documents
Questions & Answers