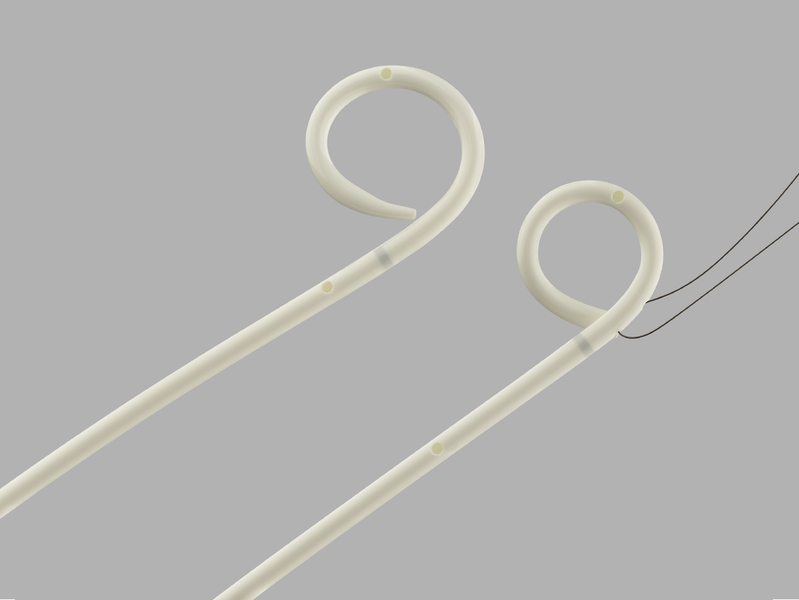
INTENDED USE
The Amplatz Ureteral Stent Set is delivered percutaneously and is intended to establish drainage from the ureteropelvic junction to the bladder and stenting of the ureter for all patients in whom it is desirable to place a drain which does not extend externally. The Amplatz Ureteral Stents are not intended to remain indwelling more than 180 days.
CONTRAINDICATIONS This device is contraindicated in the presence of conditions which create unacceptable risk during catheterization.
WARNINGS This product contains NATURAL RUBBER LATEX, which may cause allergic reactions.
PRECAUTIONS
• The product is intended for use by physicians trained and experienced in diagnostic and interventional techniques. Standard techniques for placement of ureteral scents should be employed.
• This product is intended for use in adult patients only.
• Do not over-advance ureteral stent onto stent positioner. Crimping of stent could occur.
• The potential effects of phthalates on pregnant/nursing women or children have not been fully characterized and there may be concern for reproductive and developmental effects.
• Where long-term use is indicated, it is recommended that indwelling time not exceed 180 days and that the physician evaluate the catheter before this time has expired.