Indications
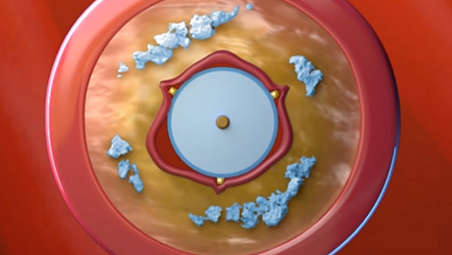
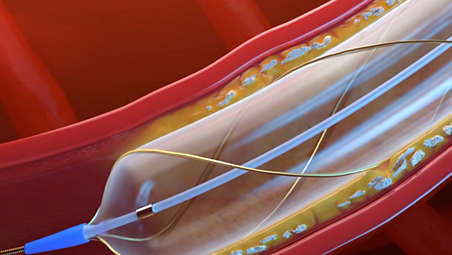
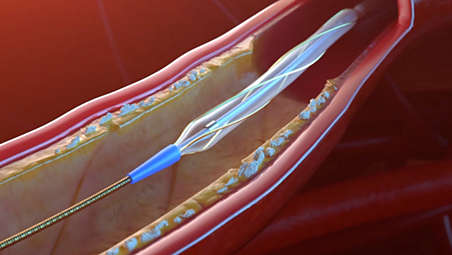
The AngioSculpt PTA scoring balloon catheter is intended for dilatation of lesions in the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. Not for use in the coronary or neuro-vasculature.
Contraindications
None known for percutaneous transluminal angioplasty (PTA) procedures.
Warnings
This device is intended for single (one) patient use only. Do not resterilize and/or reuse, as this can potentially result in compromised device performance and increased risk of inappropriate resterilization and cross contamination. The inflated diameter of the balloon should approximate the diameter of the vessel just proximal and distal to the stenosis, in order to reduce potential vessel damage. When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated under vacuum. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Balloon pressure should not exceed the rated burst pressure (RBP). Refer to product label for device-specific information. The RBP is based on results of in-vitro testing. At least 99.9% of the balloons (with a 95% confidence level) will not burst at or below their RBP. Use of a pressure monitoring device is recommended to prevent over-pressurization. Use only the recommended balloon inflation medium. Never use air or any gaseous medium to inflate the balloon. Proceed cautiously when using the AngioSculpt catheter in a freshly deployed bare metal or drug-eluting stent. The AngioSculpt catheter has not been tested for post-dilation of stents or in lesions distal to freshly deployed stents in clinical studies. Bench testing has shown no additional risk when inserting or withdrawing the AngioSculpt catheter through stents (no interference with stent struts, no retention of or damage to the AngioSculpt catheter). Use the catheter prior to the "Use Before" (expiration) date specified on the package.
Precautions
A thorough understanding of the principles, clinical applications and risks associated with PTA is necessary before using this product. Any use for procedures other than those indicated in these instructions is not recommended. The device is not recommended for use in lesions that may require inflation pressures higher than those recommended for this catheter. Do not use if package is opened or damaged. Prior to angioplasty, the catheter should be examined to verify functionality, device integrity and to ensure that its size and length are suitable for the specific procedure for which it is to be used. During and after the procedure, appropriate anticoagulants, antiplatelet agents and vasodilators should be administered to the patient according to institutional practice for peripheral angioplasty of similar arteries. Pass the AngioSculpt catheter through the recommended introducer shea