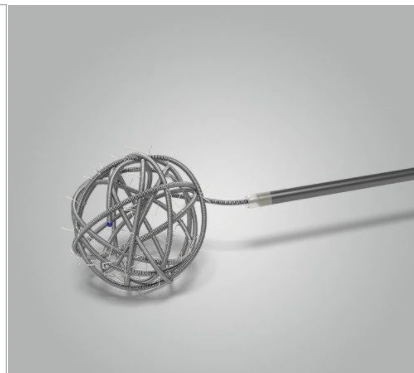
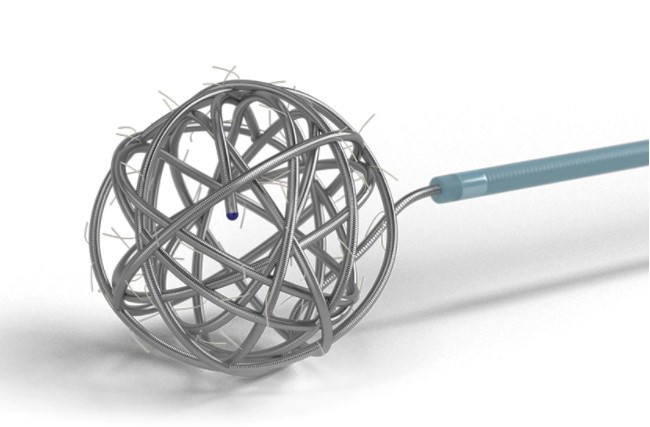
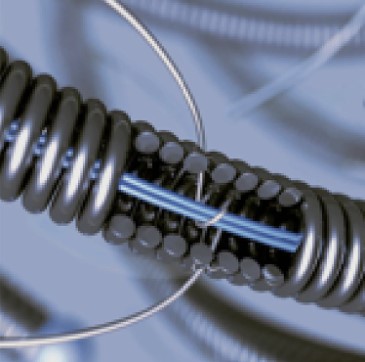
Axium™ MicroFX™ coils with LatticeFX™ technology have overlapped individual nylon or PGLA microfilaments between coil loops, forming an intra-aneurysmal surface area for stable scar tissue formation and high thrombogenicity.
These coils provide the acute performance of an Axium™ Bare coil with the added benefit of LatticeFX™ technology.
- Flow reduction across the neck.
- Increased thrombogenicity.
- 33% less flow through MicroFX™ coils vs. standard Axium™ coils.
KEY BENEFITS OF AXIUM™ MICROFX™ COILS
INTERLOCKED SURFACE AREA
Polymer fibers: Axium™ MicroFX™ coils contain PGLA or nylon microfilaments designed to impact flow reduction and accelerate thrombosis.
LatticeFX™ Technology: LatticeFX™ technology provides an interlocked surface area or scaffold which orients cell adhesion and extracellular deposition.


ACCELERATED THROMBUS FORMATION
Enhanced thrombin generation with filamented coils when compared to bare platinum coils and higher thrombogenicity may be beneficial in situations where rapid occlusion is needed.
VESSEL OR FISTULA TAKE DOWN
An alternative to bare platinum coils for arteriovenous fistulae and other neurovascular abnormalities.