Manufacturer > Stryker Neurovascular > Devices > AXS Catalyst™ Distal Access Catheter
AXS Catalyst™ Distal Access Catheter
Device-Type
Aspiration Catheter
Manufacturer
Stryker Neurovascular
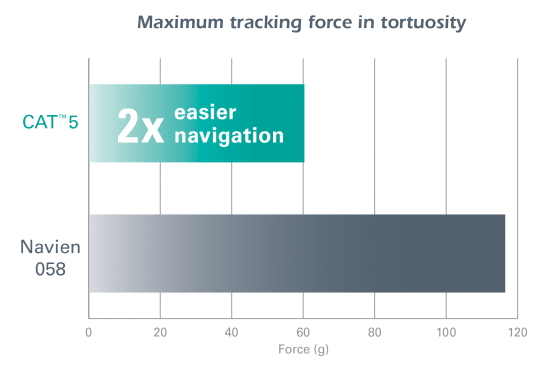
The CAT™ 5 Distal Access Catheter provides easy navigation and reliable support for distal access cases.
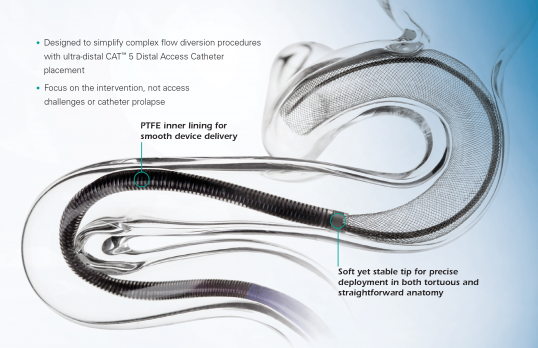
The AXS Catalyst Distal Access Catheter is a single lumen, variable stiffness catheter designed for use in facilitating the insertion and guidance of appropriately sized interventional devices into the peripheral and neuro vascular system. The catheter shaft has a hydrophilic coating to reduce friction during use. The catheter includes a radiopaque marker on the distal end for angiographic visualization and a luer hub on the proximal end allowing attachments for flushing and aspiration. It is packaged with a Rotating Hemostastic Valve (RHV) and Tuohy Borst valve with sideport for flushing, insertion of catheters and aspiration. The peel away introducer sheaths are designed to protect the distal tip of the catheter during insertion into the RHV or Tuohy Borst.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers