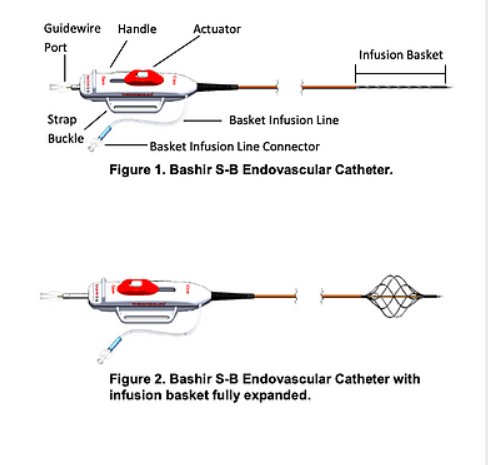
Contraindications
The Bashir S-B Endovascular Catheter is contraindicated for use in the coronary arteries, pulmonary arteries, and neurovasculature.
Precaution and Warnings
• The Bashir S-B Endovascular Catheter must only be used by physicians trained in interventional vascular procedures.
• Do not use the Bashir S-B Endovascular Catheter with a power injector as catheter damage may occur.
• This product is supplied STERILE using an ethylene oxide (EO) process. Carefully inspect the device packaging prior to use. Do not use if package appears open or damaged.
• Carefully inspect the device prior to use. Do not use the device if it appears damaged or if any of its components is missing.
• Use the device only prior to the “Use By” date listed on the package label.
• Store in a dry, cool place.
• This product is designed and intended for single use. Do not re-use.
• Do not re-sterilize.
• Re-using or re-sterilizing may be detrimental to the structural integrity and proper function of the product, resulting in patient injury or death. Reusing the product may also result in product contamination which may lead to infection and/or the transmission of infectious disease(s), resulting in patient injury, illness or death.
• Dispose of the product and package according to hospital and/or local government policies.
• Use the Bashir S-B Endovascular Catheter only with the sheath and guidewire sizes indicated in these instructions.
• The Bashir S-B Endovascular Catheter is designed to be used under standard fluoroscopic observation.
• Do not advance or manipulate the device in the vasculature if resistance is felt. Advancing or manipulating the device when resistance is felt may result in vessel trauma or device damage. If resistance is met, determine the cause of the resistance via fluoroscopy before proceeding.
• Do not apply excessive torque or rotation to the system.
• All physician-specified fluids to be infused must be used according to the manufacturer’s instructions for use.
• Flush the entire device with heparinized saline or suitable flush solution prior to placement to avoid accidental introduction of air into the system.
• Before placement, verify that the diameter of the infusion basket can be adjusted using the actuator on the handle. Moving the actuator in the proximal direction (towards the operator), increases the infusion basket diameter. Moving the actuator in the distal direction (away from the operator), while simultaneously pressing the actuator release button, reduces the infusion basket diameter.
• Do not move the handle actuator in the distal direction without simultaneously pressing the actuator release button.
• Before moving the device within a blood vessel, ensure that the infusion limbs are collapsed by moving the actuator handle in the distal direction.
• Ensure that the basket infusion connector is attached to an infusion pump with the physician-specified fluid at the rate prescribed by the physician prior to introducing the device into the vasculature and during insertion and placement. This will maintain patency of the infusion basket.
• Do not expand the infusion basket to touch the walls of the blood vessel; the infusion basket should remain within the vascular walls whether expanded or closed.