Manufacturer > Merit Medical > Devices > Bearing nsPVA® Embolization Particles
Bearing nsPVA® Embolization Particles
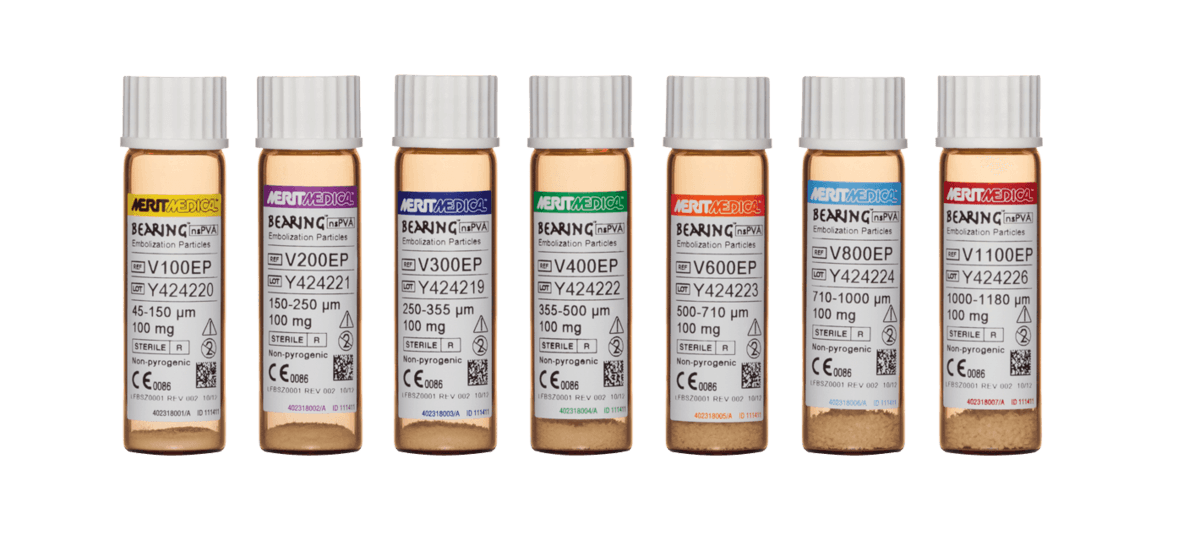
Bearing nsPVA Embolization Particles are irregularly-shaped, biocompatible, hydrophilic, nonresorbable particles produced from polyvinyl alcohol. These embolization particles are intended to provide vascular occlusion or reduction of blood flow within target vessels upon selective placement through a variety of catheters. Bearing nsPVA Embolization Particles are used for the embolization of arteriovenous malformations and hypervascularized tumors, including symptomatic uterine fibroids.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×