Manufacturer > Cardiva Medical > Devices > CATALYST Mannual Compression
CATALYST Mannual Compression
Device-Type
Closure Devices
Manufacturer
Cardiva Medical
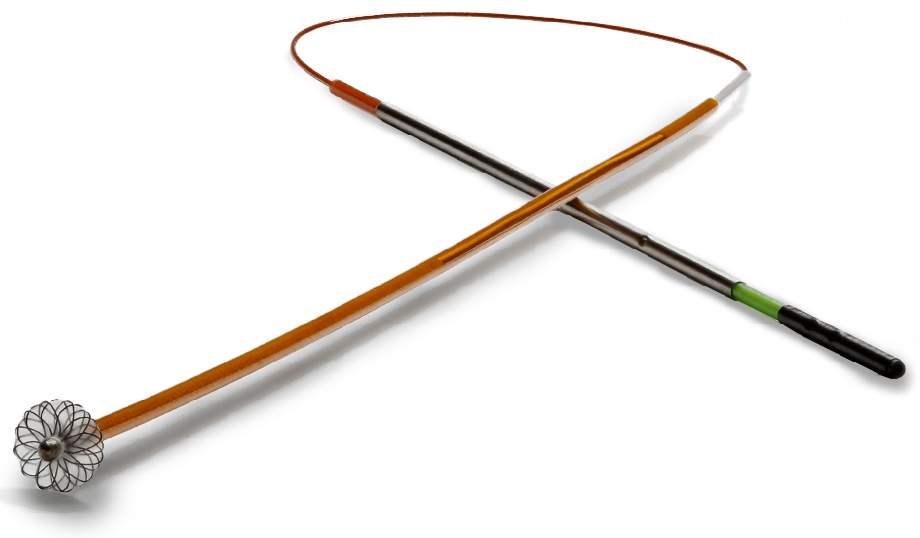
The Cardiva Catalyst™ II consists of a sterile disposable Catalyst II Wire and a sterile disposable Catalyst Clip. The Cardiva Catalyst II promotes hemostasis at a femoral access site after femoral arterial catheterization as an adjunct to manual compression. After completion of catheterization procedure, the Catalyst II Wire is inserted into the artery through the existing introducer sheath. The distal tip of the Catalyst II Wire is deployed, which opens the bi-convex, low profile Catalyst Disc within the lumen of the femoral artery distal to the introducer sheath tip. After removing the introducer sheath over the Catalyst II Wire, gentle upward tension is applied to the Catalyst II Wire to conform the Catalyst Disc to the contours of the vessel securing it against the intima, blocking the arteriotomy. Tension is maintained by applying the Catalyst Clip externally to the Catalyst II Wire at the puncture site. Tension between the Catalyst Disc and the Catalyst Clip creates a site-specific compression of the arteriotomy and establishes temporary hemostasis. During dwell, natural recoil of smooth muscle in the vessel wall occurs at the arteriotomy site. A biocompatible coating on the Catalyst II Wire aides the body’s natural hemostatic process and promotes ease of removal. Following appropriate dwell time, the Catalyst Disc is collapsed and the Cardiva Catalyst II is completely removed from the artery. No part of the device is left behind. Final hemostasis of the vessel puncture site occurs with application of manual or mechanical compression after removing the Cardiva Catalyst II.
The Cardiva Catalyst III consists of a sterile disposable Catalyst III Wire and a sterile disposable Catalyst Clip. The Cardiva Catalyst III is specifically designed for use with heparinized patients. The Cardiva Catalyst III promotes hemostasis at a femoral access site after femoral arterial catheterization as an adjunct to manual compression in heparinized patients. After completion of catheterization procedure, the Catalyst III Wire is inserted into the artery through the existing introducer sheath. The distal tip of the Catalyst III Wire is deployed, which opens the bi-convex, low profile Catalyst Disc within the lumen of the femoral artery distal to the introducer sheath tip. After removing the introducer sheath over the Catalyst III Wire, gentle upward tension is applied to the Catalyst III Wire to conform the Catalyst Disc to the contours of the vessel securing it against the intima, blocking the arteriotomy. Tension is maintained by applying the Catalyst Clip externally to the Catalyst III Wire at the puncture site. Tension between the Catalyst Disc and the Catalyst Clip creates a site-specific compression of the arteriotomy and establishes temporary hemostasis. During dwell, natural recoil of smooth muscle in the vessel wall occurs at the arteriotomy site. A biocompatible coating on the Catalyst III Wire aides the body’s natural hemostatic process and promotes ease of removal. Additionally, the Cardiva Catalyst III biocompatible coating includes a minimal amount of Protamine Sulfate to locally neutralize heparin to further aid the body’s natural hemostatic process in heparinized patients. Following appropriate dwell time, the Catalyst Disc is collapsed and the Cardiva Catalyst III is completely removed from the artery. No part of the device is left behind. Final hemostasis of the vessel puncture site occurs with application of manual or mechanical compression after removing the Cardiva Catalyst III.
INDICATIONS
The Cardiva Catalyst II System is intended to promote hemostasis at arteriotomy sites as an adjunct to manual compression. The Cardiva Catalyst II is indicated for use in patients undergoing diagnostic and/or interventional femoral artery catheterization procedures, using 5F, 6F or 7F introducer sheaths.
The Cardiva Catalyst III System with Protamine Sulfate is intended to promote hemostasis at arteriotomy sites as an adjunct to manual compression in heparinized patients. The Cardiva Catalyst III is indicated for use in patients undergoing diagnostic and/or interventional femoral artery catheterization procedures, using 5F, 6F or 7F introducer sheaths.
CATALYST is designed to provide temporary hemostasis and enhance coagulation for patients requiring manual compression – helping to reduce mean hold time by over 50% and reduce time to ambulation by more than 20% as compared to manual compression.2
- Enhances tissue apposition, natural elastic recoil and coagulation
- Helps maintain vessel integrity
- Supports the body’s natural healing process
- Allows immediate reaccess with no part of the device left behind
- More efficient for clinical staff and more comfortable for patients
CATALYST Activates Clotting and Promotes Coagulation
The CATALYST II and CATALYST III manual compression assist devices employ Cardiva’s proven proprietary collapsible disc technology and two different hemostatic coatings based on the presence of heparin. CATALYST II is coated with kaolin and chitosan used to promote coagulation by activating the clotting cascade and causing platelet aggregation. CATALYST III is coated with an additional drug, protamine sulfate, acting locally to neutralize heparin and further aid the body’s natural healing process.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers