CONTRAINDICATIONS
There are no known contraindications for this device.
WARNINGS
Do not use Closer* VSS if the package/device is damaged, if any portion of the package has been previously opened, or if any item appears defective in any way.
Do not use Closer* VSS if the temperature indicator dot has changed from light grey to dark grey/black.
Do not resterilize or re-use the Closer* VSS in any manner; it is for SINGLE USE ONLY.
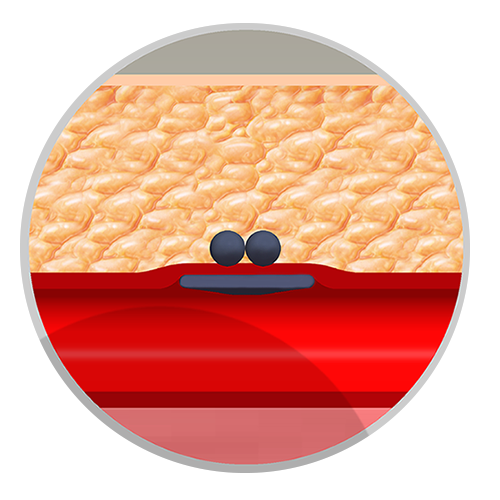
Do not use Closer* VSS if the posterior wall of the artery is punctured, or if the puncture site is a) proximal to the inguinal ligament, or b) at or distal to the bifurcation of the superficial femoral and profunda femoris artery, as this may result in 1) the intravascular sealing patch catching on the bifurcation or being positioned incorrectly, and/or 2) intravascular deployment of the device into the vessel (These 2 events may reduce blood flow through the vessel leading to symptoms of distal arterial insufficiency.) or 3) extravascular deployment of the device outside of the vessel (This event may result in retroperitoneal bleeding).
Do not use Closer* VSS if the sterile field has been broken or where bacterial contamination of the procedural sheath or surrounding tissues may have occurred as this may increase risk of infection.
Do not use in patients with known allergies to polylactic acid (PLA), polyglycolic acid (PGA), or polydioxanone (PDO) polymers.
PRECAUTIONS
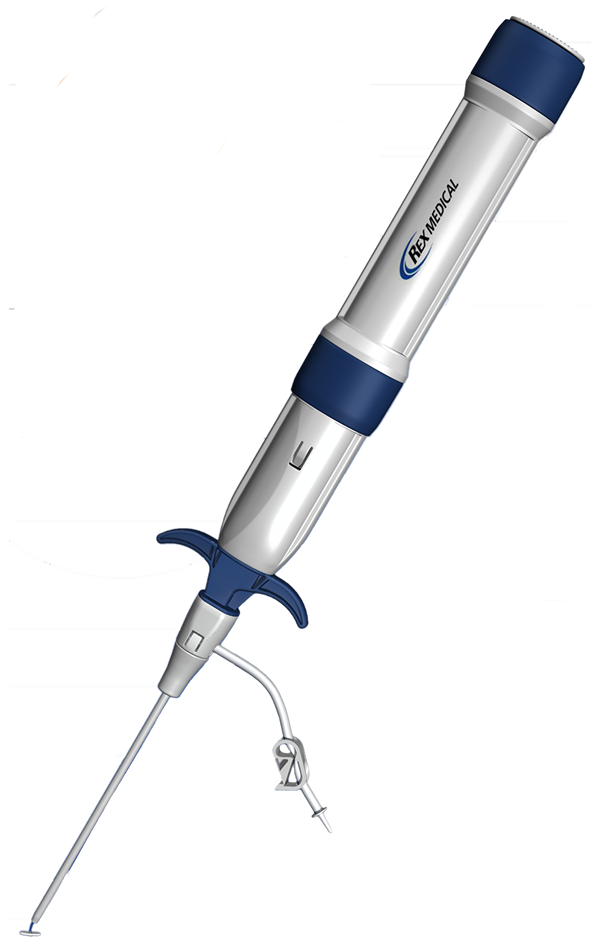
Do not use the Closer* Delivery System with any sheath other than the insertion sheath provided in the kit. Use only the Closer* Access Kit (insertion sheath/dilator combination) provided to locate the puncture in the arterial wall.
The Closer* VSS should be used within one hour of opening the foil pouch.
If there is suspicion that the Closer* Delivery System patch may not be seated against the intimal aspect of the arteriotomy site, the system components and delivery system should be simultaneously withdrawn from the patient. Hemostasis can then be achieved by applying manual pressure or other closure technique as directed by the physician.
If femoral access is required within 90 days, it should be attempted on the contralateral side. If this is not possible, and re‐ puncture on the original treatment side is required, access the femoral artery at least 2 cm proximal to the original puncture site.
Disposal of contaminated device, components, and packaging materials will be according to universal precautions for biohazardous waste.
The Closer* VSS should only be used by a trained licensed physician or healthcare professional.