Manufacturer > Boston Scientific > Devices > EkoSonic™ Endovascular System
EkoSonic™ Endovascular System
Device-Type
Mechanical Thrombectomy
Manufacturer
Boston Scientific
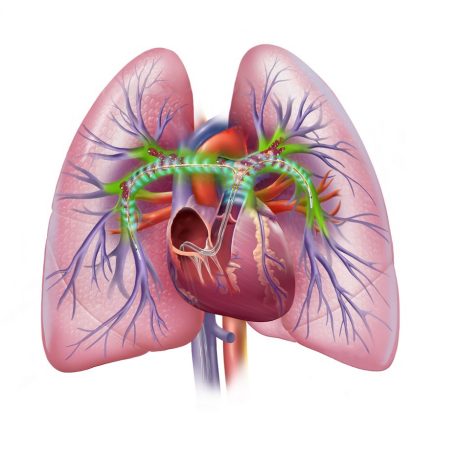
EKOS was the first product indicated for the treatment of pulmonary embolism. The EkoSonic catheter can be used for the infusion of physician selected therapeutics, including thrombolytics into the peripheral vasculature.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×