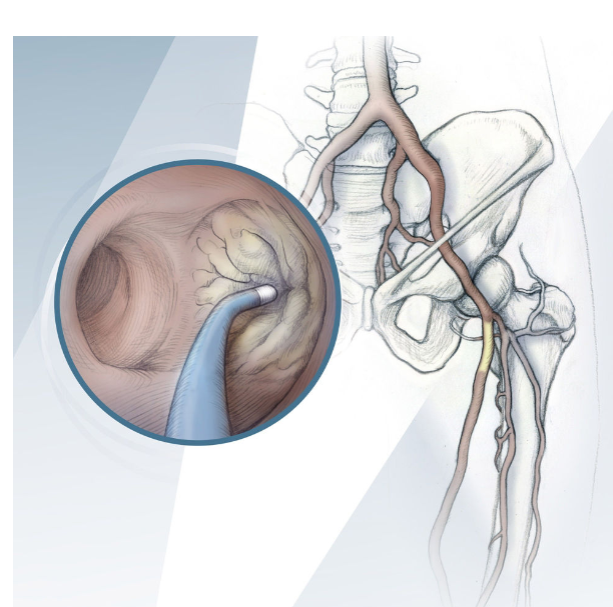
The ELITECROSS™ Support Catheter provides a unique combination of support and shape options to enable a tailored approach to tough lesions.
Features and Benefits
Safety informations
Device Documents
Questions & Answers
×