Device-Type
Drug-Eluting Stents
Targated Speciality
Vascular
Manufacturer
Boston Scientific
Used in Procedure
Any Procedure
Eluvia provides consistent, durable outcomes in challenging SFA disease and features a polymer design for controlled drug release.
Exceptional Outcomes in Complex Lesions
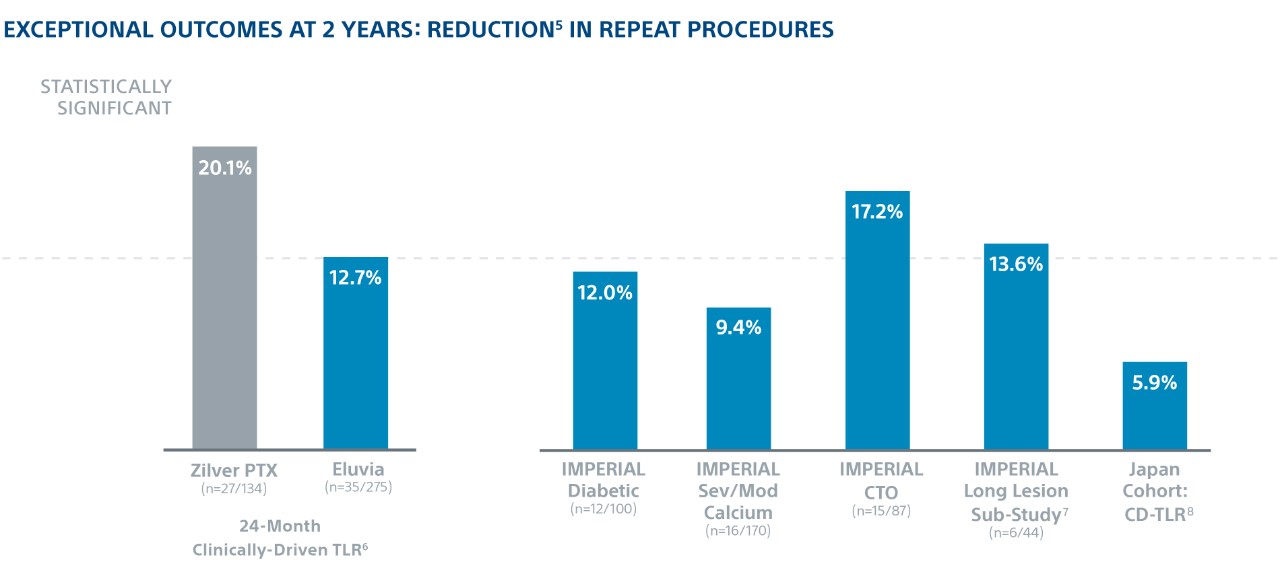
In the world’s first head-to-head DES SFA trial1, Eluvia demonstrated the highest ever reported 2-year Kaplan-Meier primary Patency of 83%2 and showed low 2-year TLR in patients with long lesions, diabetes, CTOs, and severe/moderate calcium.
As the only device using polymer to elute drug for PAD, Eluvia features the lowest drug dose with the fewest downstream particulates3, leaving no paclitaxel in the bloodstream at 30 minutes post implant.4
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×