CONTRAINDICATIONS:
• Patients intolerant to occlusion procedures
• Vascular anatomy or blood flow that precludes catheter placement or embolic agent injection.
• Presence or likely onset of vasospasm.
• Presence or likely onset of hemorrhage.
• Presence of severe atheromatous disease.
• Presence of feeding arteries smaller than distal branches from which they emerge. • Presence of patent extra-to-intracranial anastomoses or shunts.
• Presence of collateral vessel pathways potentially endangering normal territories during embolization.
• Presence of end arteries leading directly to cranial nerves.
• Presence of arteries supplying the lesion not large enough to accept EmboGold Microspheres.
• Vascular resistance peripheral to the feeding arteries precluding passage of EmboGold Microspheres into the lesion.
• Do not use EmboGold Microspheres in the following applications: a. Embolization of large diameter arteriovenous shunts (i.e. where the blood does not pass through an arterial/capillary/venous transition but directly from an artery to a vein). b. In the pulmonary arterial vasculature. c. Use in any vasculature where EmboGold Microspheres could pass directly into the internal carotid artery, vertebral artery, intracranial vasculature or the above-listed vessels.
• Patients who have an allergic response to gold.
PRECAUTION:
• Do not use if the syringe, plunger seal or tray package appears damaged.
• For single patient use only - Contents supplied sterile - Never reuse, reprocess, or resterilize the contents of a syringe that has beeN opened. Reusing, reprocessing or resterilizing may compromise the structural integrity of the device and or lead to device failure, which in turn may result in patient injury, illness or death. Reusing, reprocessing or resterilizing may also create a risk of contamination of the device and or cause patient infection or cross infection including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient. All procedures must be performed according to accepted aseptic technique.
• Do not connect the 20 mL syringe with EmboGold Microspheres directly to a microcatheter for embolic delivery, as a catheter occlusion may result.
• The syringe is intended for embolic use only. Do not use for another application.
• Select the size and quantity of EmboGold Microspheres appropriate for the pathology to be treated.
• Embolization with EmboGold Microspheres should only be performed by physicians who have received appropriate interventional occlusion training in the region intended to be embolized.
• Patients with known allergy to contrast medium may require corticosteroids prior to embolization.
• Additional evaluations or precautions may be necessary in managing periprocedural care for patients with the following conditions: – Bleeding diathesis or hypercoagulative state – Immunocompromise.
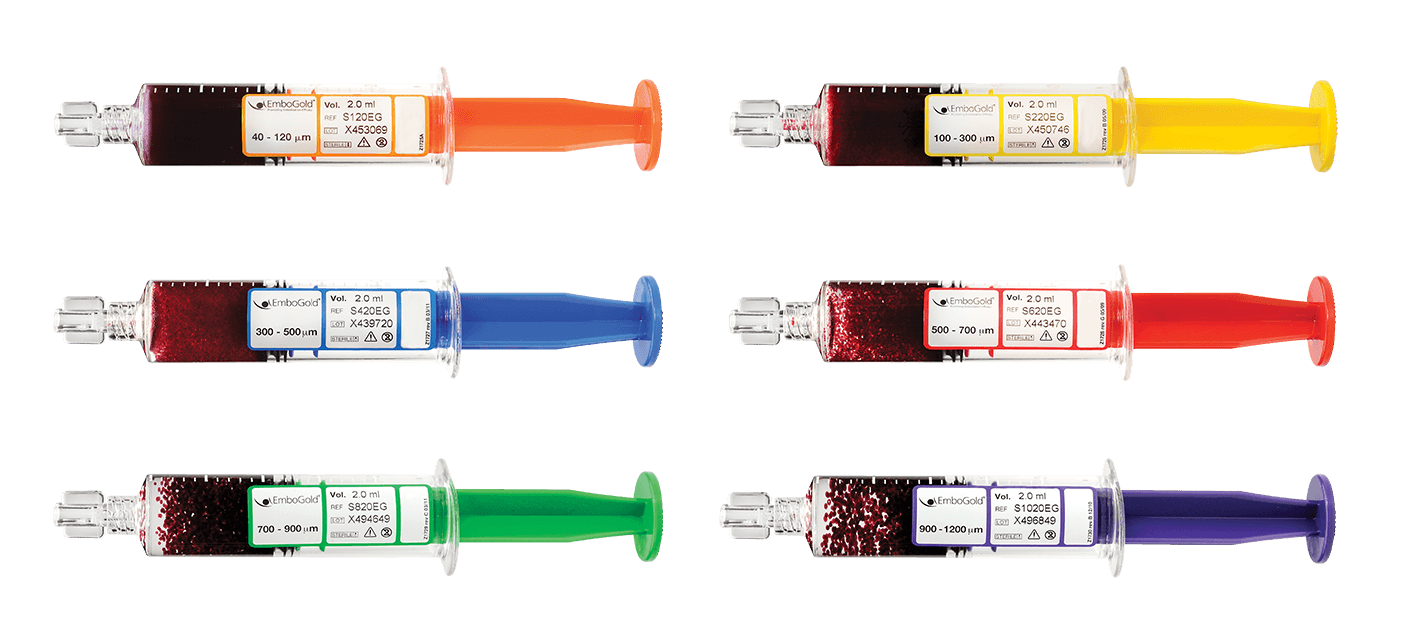
 PVA
PVA Embogold Microspheres
Embogold Microspheres