Manufacturer > Merit Medical > Devices > Embosphere PRO™ Prostatic Artery Embolization Kit
Embosphere PRO™ Prostatic Artery Embolization Kit
Device-Type
Embolic Particles/Beads
Manufacturer
Merit Medical
Prostatic artery embolization (PAE) is a technically challenging procedure that demands a predictable, targeted, and established microsphere like Embosphere® Microspheres.
The first and only embolic cleared by the U.S. Food & Drug Administration for PAE, Embosphere® Microspheres are available in Merit’s exclusive Embosphere PRO™ Prostatic Artery Embolization Kit, which brings additional convenience to the power and predictability of Embosphere® Microspheres.
CONVENIENT
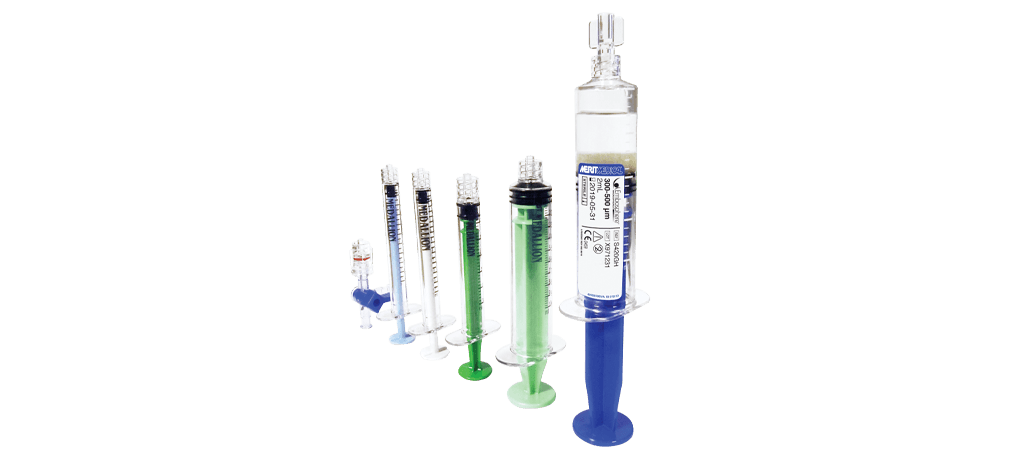
Merit Medical’s new Embosphere PRO™ Prostatic Artery Embolization Kit combines the power and predictability of Embosphere® Microspheres with basic preparation and delivery tools for added convenience.
Each kit includes:
- (1) prefilled syringe of Embosphere® Microspheres, 300-500μm or 100-300μm
- (1) 10 mL Medallion® Syringe
- (1) 3 mL Medallion® Syringe
- (2) 1 mL Medallion® Syringes
- (1) 3-way Medallion® Stopcock, 1050 psi
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×