IMPORTANT SAFETY INFORMATION
 ENGAGE™ INTRODUCER / ENGAGE™ TR INTRODUCER Hemostasis Introducer
ENGAGE™ INTRODUCER / ENGAGE™ TR INTRODUCER Hemostasis Introducer
APPLICATIONS
The Engage™ Introducer is indicated for the introduction of angiographic catheters, closed end catheters, balloon catheters, and electrodes into a blood vessel (including but not limited to femoral, radial, and brachial access) where minimizing blood loss is essential.
The Engage™ TR Introducer is indicated for the introduction of angiographic catheters, closed end catheters, balloon catheters, and electrodes into a radial vessel where minimizing blood loss is essential.
CAUTIONS
- DO NOT alter this device.
- Federal law (U.S.) restricts this device to sale by or on the order of a physician.
- Do not attempt to insert a catheter having a distal tip or body size larger than the introducer size indicated.
- Do not attempt to use a guidewire over maximum diameter specified on package label.
- This device should only be used by physicians thoroughly trained in the technique of angiography and/or the use of St. Jude Medical catheter delivery systems. catheters
- Individual patient anatomy and physician technique may require procedural variations.
- The closing force of the hemostasis valve may alter or impair the function of some catheters.
- Damage to guidewire may result if with-drawn through a metal needle cannula. Metal needle must be removed first.
- Do not attempt to advance or withdraw guidewire if resistance is felt. Use fluo-roscopy to determine cause.
- Damage to the valve assembly may occur if inner catheter is withdrawn rapidly.
- Advance dilator/sheath assembly with a twisting motion to avoid damage to the sheath or vessel.
- For Single Use Only! Single-use devices are designed and tested for only one patient application. These are disposable devices and are not designed for repro-cessing and reuse. Reuse of designated “single-use” devices creates a risk of patient or user infections due to prior patient use and the difficulty in cleaning the narrow structures at material interfaces following direct blood contact. Contamination or reprocessing cleaning agent residues may lead to adverse patient reactions and may damage the device. Use of non-St. Jude Medical packaging may compromise device functionality and sterility due to com-promised protection from shipping and handling damage. The absence of labeling after reprocessing, may lead to misuse of the device and impaired traceability. Reprocessing and reuse may result in patient or user injury, permanent impair-ment, or death
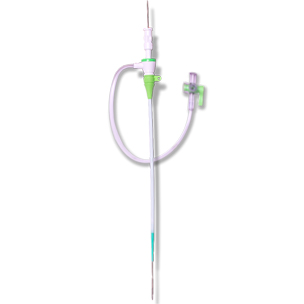


 ENGAGE™ INTRODUCER / ENGAGE™ TR INTRODUCER Hemostasis Introducer
ENGAGE™ INTRODUCER / ENGAGE™ TR INTRODUCER Hemostasis Introducer