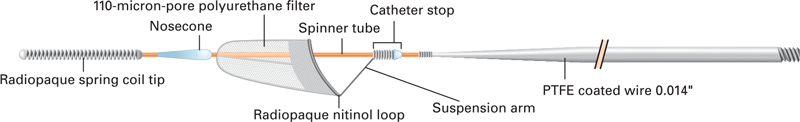
INTENDED USE/INDICATIONS FOR USE
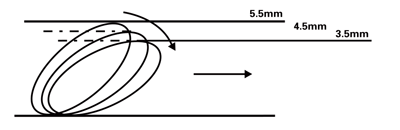
The Boston Scientific FilterWire EZ™ Embolic Protection System is indicated for use as a guidewire and embolic protection system to contain and remove embolic material (thrombus/debris) while performing angioplasty and stenting procedures in coronary saphenous vein bypass grafts and carotid arteries. The diameter of the vessel at the site of filter loop placement should be between 2.25 mm and 5.5 mm for coronary saphenous vein bypass graft procedures and between 3.5 mm and 5.5 mm for carotid procedures.
The safety and effectiveness of this device as an embolic protection system have not been established in the cerebral vasculature, peripheral vessels other than carotid arteries, or in treating native coronaries, including acute myocardial infarction.
CONTRAINDICATIONS
- Patients with severe allergy to heparin
- Patients with bleeding diathesis or other disorders that limit the use of anticoagulant therapy
WARNINGS
- Only physicians thoroughly trained in percutaneous intravascular techniques and procedures should use the FilterWire EZ Embolic Protection System (refer to Physician Training section).
- The safety and effectiveness of coronary drug-eluting stents (DES) when used with embolic protection devices have not been established.
- The safety and effectiveness of the FilterWire EZ Embolic Protection System have not been demonstrated with carotid stents other than the Carotid WALLSTENT® Monorail® Endoprosthesis and Delivery System (Carotid WALLSTENT Endoprosthesis) and the NexStent® Carotid Stent and Monorail Delivery System (NexStent Carotid Stent).
- The appropriate antiplatelet and anticoagulation therapy should be administered pre- and post-procedure to minimize the risk of embolism and thrombosis.
- Filter safety and effectiveness has not been tested with power injection.
- Failure to follow recommended device preparation and delivery instructions may result in air embolism or other adverse reaction.