INDICATIONS FOR USE
FlowGate2™ Balloon Guide Catheters are indicated for use in facilitating the insertion and guidance of an intravascular catheter into a selected blood vessel in the peripheral and neuro vascular systems. The balloon provides temporary vascular occlusion during these and other angiographic procedures. The Balloon Guide Catheter is also indicated for use as a conduit for Retrieval devices.
COMPATIBILITY
Introducer sheath French size must be greater than or equal to balloon guide catheter French size.
WARNINGS
•Do not reuse. Discard after one procedure. Structural integrity and/or function may be impaired through reuse or cleaning.
•Never advance or torque catheter against resistance without careful assessment of cause of resistance using fluoroscopy. If cause cannot be determined, withdraw catheter. Movement against resistance may result in damage to vessel or catheter.
•To reduce risk of complications due to slow balloon deflation, adhere to the following recommendations: – Wet distal shaft with saline before advancing peel-away sheath over balloon. – Use peel-away sheath to advance catheter into introducer sheath. – Minimize pushing forces on shaft during advancement. These forces can cause wrinkles in shaft that can slow balloon deflation. – Do not use device if shaft is damaged during use. – Prepare balloon according to Recommended Procedure.
•To reduce risk of complications due to air emboli, remove air from balloon according to Recommended Procedure.
•Withdrawing balloon through introducer sheath may damage balloon. Do not use catheter again after withdrawing balloon through introducer sheath.
•To avoid balloon leakage, do not allow balloon to contact calcified or stented arteries and do not allow balloon to move during inflation.
•Do not use a device that has been damaged. Use of damaged devices may result in complications.
•Do not exceed maximum recommended balloon inflation volume. Excess inflation volume may rupture balloon.
•For through-lumen, do not exceed 2068 kPa (300 psi) maximum recommended infusion pressure. Excess pressure may result in catheter rupture or tip detachment.
•If flow through catheter becomes restricted, do not attempt to clear catheter lumen by infusion. Doing so may cause catheter to rupture, resulting in vessel trauma. Remove and replace catheter.
•Do not steam shape guide catheter.
PRECAUTIONS
•Store in a cool, dry, dark place.
•Do not use open or damaged packages.
•Use by “Use By” date.
•Exposure to temperatures above 54°C (130°F) may damage device and accessories. Do not autoclave.
•Upon removal from package, inspect device to ensure it is not damaged.
•Do not expose device to solvents.
•Use device in conjunction with fluoroscopic visualization and proper anti-coagulation agents.
•Torquing guide catheter while kinked may cause damage that could result in separation of catheter shaft.
•If a device becomes lodged in guide catheter, or if guide catheter becomes severely kinked, withdraw entire system (guide catheter, guidewire and catheter sheath introducer).
•To prevent thrombus formation and contrast media crystal formation, maintain a constant infusion of appropriate flush solution through guide catheter lumen.

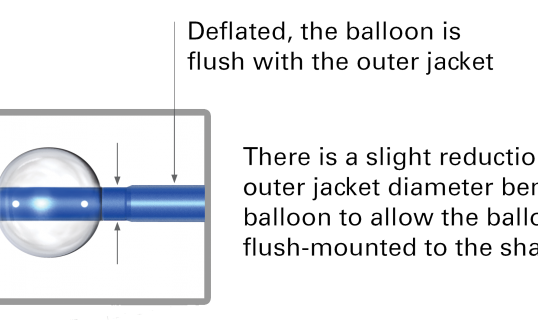
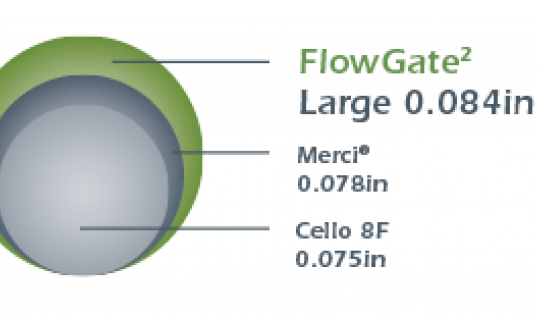
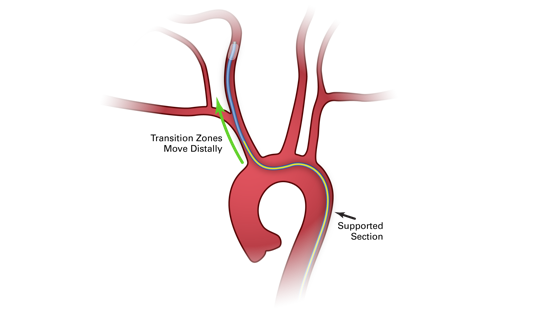







_538_320_s_c1.png)



