Manufacturer > Cook Medical > Devices > Formula 418® Biliary Balloon-Expandable Stent
Formula 418® Biliary Balloon-Expandable Stent
Device-Type
Balloon-Expandable Stents
Targated Speciality
Endovascular
Disease Solution
Peripheral Arterial Disease (PAD)
Manufacturer
Cook Medical
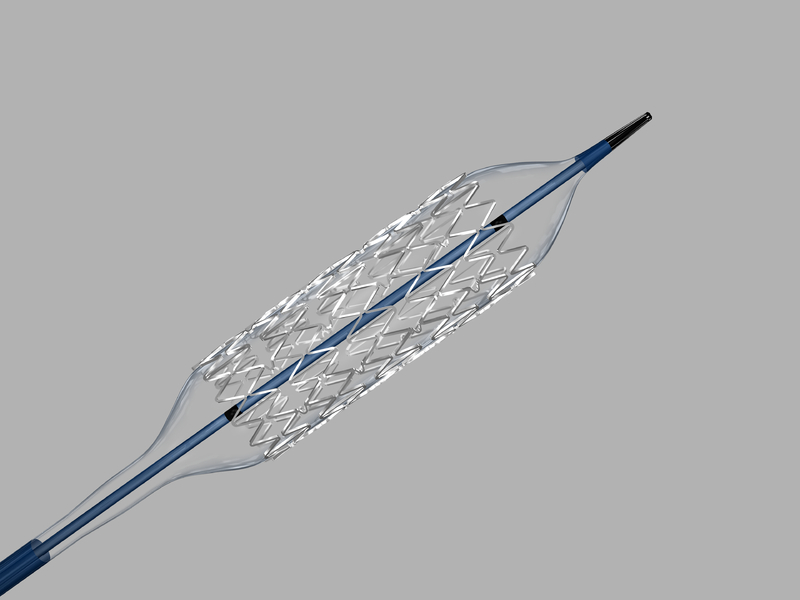
The Formula 418 Stent is a balloon-expandable stent made of 316L stainless steel with a slotted tube configuration. It is pre-mounted on a balloon catheter, which serves as the delivery system. The stent is positioned between two radiopaque marker bands, which are located inside the balloon at the proximal and distal tapers of the balloon. The cannula design of the Formula 418 Stent provides a low outside diameter profile and is pre-mounted on either an 80 cm or 135 cm balloon catheter delivery system. The stent is available in nominal expanded diameters ranging from 3-8 mm with lengths ranging from 12-30 mm.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×