Device-Type
Flow Diverter
Targated Speciality
Endovascular
Disease Solution
AneurysmTherapy
Manufacturer
MicroVention Terumo
Used in Procedure
Angioplasty
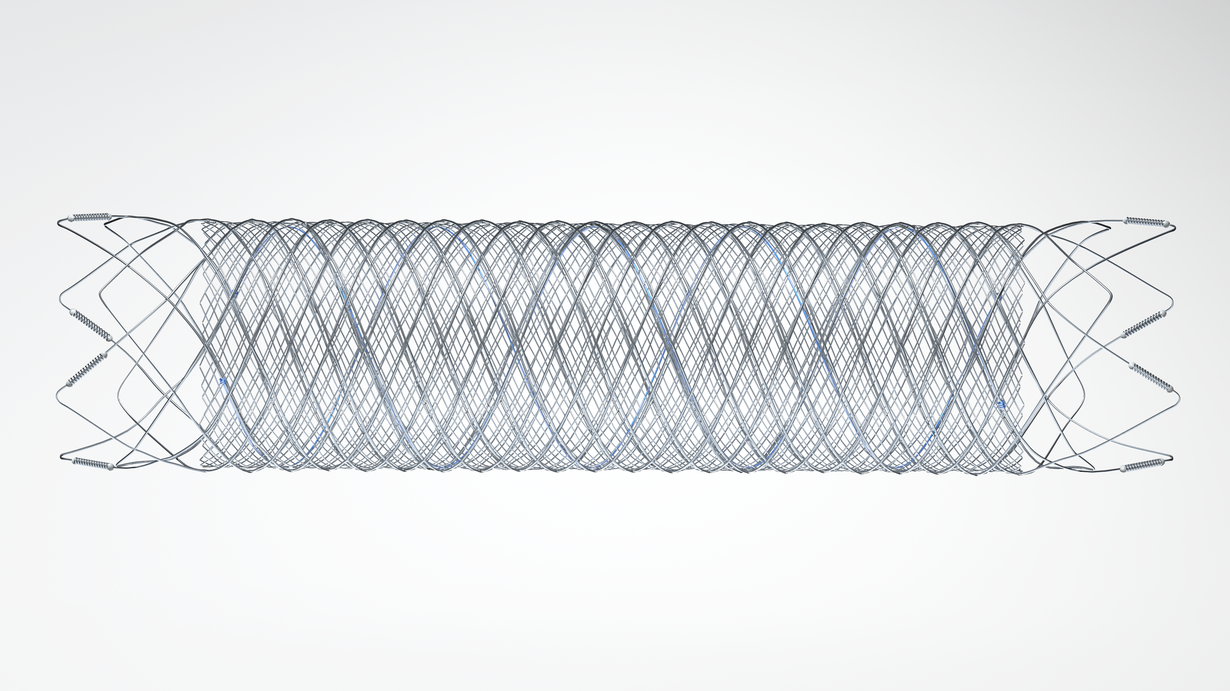
The FRED™ device is the first flow diverter in the U.S. to use a self-expanding braided nitinol mesh to help re-direct blood flow and promote aneurysm occlusion. The unique interwoven nitinol design of the FRED™ device allows for smooth delivery to the target aneurysm, as well as reliable opening and vessel wall apposition, resulting in high treatment durability.
The FRED™ device has been CE marked since 2013, safely used in nearly 20,000 procedures, and published in numerous clinical studies around the world. The FRED™ device pivotal study adds new evidence to the large existing body of global clinical data, further demonstrating the safety and effectiveness of the device.
The Flow Re-Direction Endoluminal Device (FRED®) System is indicated for use in the internal carotid artery from the petrous segment to the terminus for the endovascular treatment of adult patients (22 years of age or older) with wide-necked (neck width ≥ 4 mm or dome-to-neck ratio < 2) saccular or fusiform intracranial aneurysms arising from a parent vessel with a diameter ≥ 2.0 mm and ≤ 5.0 mm.
Features and Benefits
Recommended Compatible Devices
Questions & Answers