Device-Type
Hybrid Vascular Graft
Manufacturer
Merit Medical
The HeRO Graft is a hemodialysis access graft for patients who are failing fistulas or grafts or are catheter-dependent due to the blockage of veins leading to the heart.
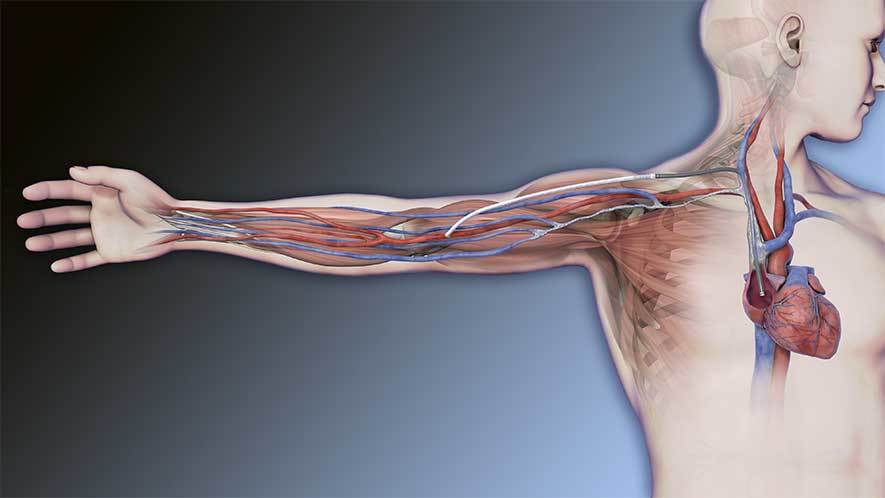
HeRO Graft (Hemodialysis Reliable Outflow) is the ONLY fully subcutaneous AV access solution clinically proven to maintain long-term access for hemodialysis patients with central venous stenosis. HeRO Graft is classified by the FDA as a graft, but differs from a conventional AV graft since it has no venous anastomosis. It consists of two primary components:
A proprietary ePTFE Arterial Graft Component
The HeRO Graft Arterial Graft Component has a 6mm inner diameter (ID), 7.4mm outer diameter (OD), and is 53cm long, inclusive of the connector. It consists of an ePTFE hemodialysis graft with PTFE beading to provide kink resistance near the proprietary titanium connector. The titanium connector attaches the Arterial Graft Component to the Venous Outflow Component. The Arterial Graft Component is cannulated using standard technique according to KDOQI guidelines.
A proprietary Venous Outflow Component
The HeRO Graft Venous Outflow Component has a 5mm ID, 19F (6.3mm) OD, and is 40cm long. It consists of radiopaque silicone with braided nitinol reinforcement (for kink and crush resistance) and a radiopaque marker band at the distal tip.
Features and Benefits
Device Documents
Questions & Answers