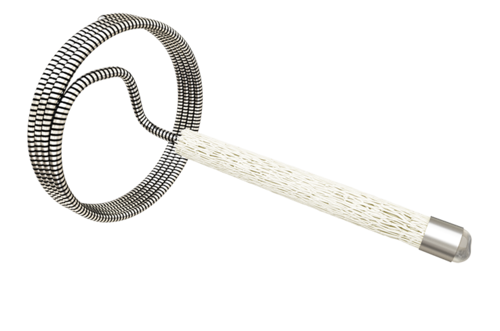
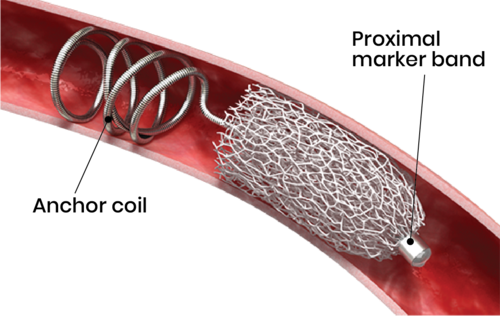
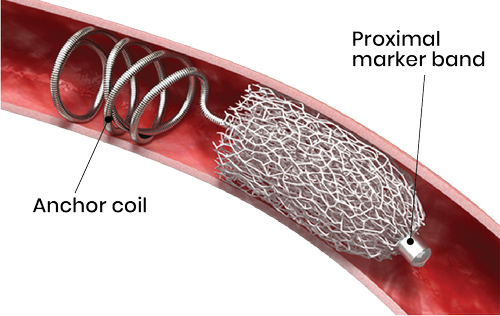
The IMPEDE Embolization Plug comprises a shape memory polymer plug and an anchor coil. The porous embolic scaffold supports the rapid formation of small interconnected clots throughout its structure. The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion. The anchor coil offers stability in higher-flow locations and IMPEDE may be used in combination with IMPEDE-FX to minimize artifact in a single vessel.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×