Caution: Federal (USA) law restricts this device to sale by or on the order of a physician. Prior to use, please refer to the Instructions for Use for complete product indications, contraindications, warnings, precautions, potential adverse events, and detailed instructions for use.
Indication For Use
INDIGO Aspiration Catheters and Separators:
As part of the INDIGO Aspiration System, the INDIGO Aspiration Catheters and Separators are indicated for the removal of fresh, soft emboli and thrombi from vessels of the peripheral arterial and venous systems, and for the treatment of pulmonary embolism.
INDIGO Aspiration Tubing:
As part of the INDIGO Aspiration System, the INDIGO Sterile Aspiration Tubing is indicated to connect the INDIGO Aspiration Catheters to the Penumbra Aspiration Pump.
Penumbra Aspiration Pump:
The Penumbra Aspiration Pump is indicated as a vacuum source for Penumbra Aspiration Systems.
Contraindications
Not for use in the coronaries or the neurovasculature.
Warnings
• The INDIGO Aspiration System should only be used by physicians who have received appropriate training in interventional techniques.
• Do not advance, retract or use any component of the INDIGO System against resistance without careful assessment of the cause using fluoroscopy. If the cause cannot be determined, withdraw the device or system as a unit. Unrestrained torquing or forced insertion of the catheter or SEPARATOR against resistance may result in damage to the device or vessel.
• Do not use the INDIGO Aspiration System with a pump other than the Penumbra Aspiration Pump.
• Placing guidewire too distal in the pulmonary vasculature or excessive manipulation of aspiration/guiding catheter in the smaller, peripheral, and segmental pulmonary artery branches can result in vessel perforation.
Precautions
• The device is intended for single use only. Do not resterilize or reuse.
• Do not use kinked or damaged devices. Do not use open or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use the INDIGO Aspiration System in conjunction with fluoroscopic visualization.
• Maintain a constant infusion of appropriate flush solution.
• When performing aspiration, ensure that the INDIGO Aspiration Tubing is open for only the minimum time needed to remove thrombus. Excessive aspiration or failure to close the INDIGO Aspiration Tubing when aspiration is complete is not recommended.
• Hemoglobin and hematorcrit levels should be monitored in patients with >700 mL blood loss from the clot aspiration procedure.
• The INDIGO SEPARATOR is not intended for use as a guidewire. If repositioning of the INDIGO Aspiration Catheter is necessary during the revascularization procedure, such repositioning should be performed over an appropriate guidewire using standard catheter and guidewire techniques.
• Do not use automated high-pressure contrast injection equipment with the INDIGO Aspiration Catheter because it may damage the device.
Potential Adverse Events
Possible complications include, but are not limited to, the following: allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arrhythmia; arteriovenous fistula; cardiac injury; cardio-respiratory arrest; death; device malfunction; distal embolization; emboli; excessive blood loss; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation; intimal disruption; myocardial infarction; emergent surgery; fibrillation; hypotension; hemoptysis; respiratory failure; thromboembolic events.
Indication For Use
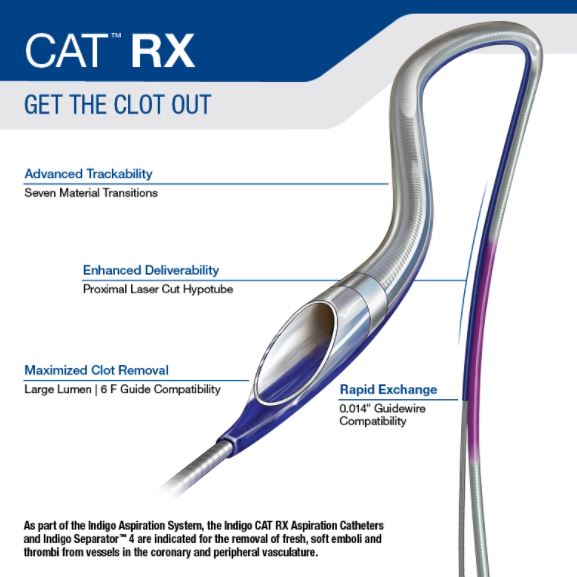
The Indigo CAT RX Aspiration Catheters and Indigo Separator 4:
As part of the Indigo Aspiration System, the Indigo CAT RX Aspiration Catheters and Indigo Separator 4 are indicated for the removal of fresh, soft emboli and thrombi from vessels in the coronary and peripheral vasculature.
The INDIGO Aspiration Tubing:
As part of the Indigo Aspiration System, the Indigo Sterile Aspiration Tubing is indicated to connect the Indigo CAT RX Aspiration Catheters to the Penumbra Aspiration Pump.
Penumbra Aspiration Pump:
The Penumbra Aspiration Pump is indicated as a vacuum source for Penumbra Aspiration Systems.
Contraindications
The Indigo Aspiration System is contraindicated in:
• The removal of fibrous, adherent or calcified material (e.g. chronic clot, atherosclerotic plaque)
• The cerebral vasculature
Warnings
• The Indigo Aspiration System should only be used by physicians who have received appropriate training in interventional techniques.
• Do not advance, retract or use any component of the Indigo Aspiration System against resistance without careful assessment of the cause using fluoroscopy. If the cause cannot be determined, withdraw the device or system as a unit. Unrestrained torquing or forced insertion of the catheter or separator against resistance may result in damage to the device or vessel.
• Do not use the Indigo Aspiration System with a pump other than the Penumbra Aspiration Pump.
Precautions
• The safety and effectiveness of this device for use in the treatment of ST-Elevation Myocardial Infarction (STEMI) has not been established. Complications from the use of this device in this manner could lead to death, permanent impairment, and/or the need for emergency medical intervention.
• The device is intended for single use only. Do not resterilize or reuse. Resterilization and/or reuse may result in ineffective catheter coating lubrication, which may result in high friction and the inability to access the target vasculature location.
• Do not use kinked or damaged devices. Do not use open or damaged packages. Return all damaged devices and packaging to the manufacturer/distributor.
• Use prior to the “Use By” date.
• Use the Indigo Aspiration System in conjunction with fluoroscopic visualization.
• Maintain a constant infusion of appropriate flush solution.
• When performing aspiration, ensure that the Indigo Aspiration Tubing valve is open for only the minimum time needed to remove thrombus. Excessive aspiration or failure to close the Indigo Aspiration Tubing valve when aspiration is complete is not recommended.
• The Indigo Separator 4 is not intended for use as a guidewire. If repositioning of the Indigo CAT RX Aspiration Catheter is necessary during the revascularization procedure, such repositioning should be performed over an appropriate guidewire using standard guidewire techniques.
• Do not use Indigo Separator 4 to macerate or retrieve thrombus distal to the catheter tip. Indigo Separator 4 is intended to be used with Indigo CAT RX Aspiration Catheter to clear the distal end of the catheter lumen should it be blocked with thrombus.
• Do not use automated high-pressure contrast injection equipment with the Indigo CAT RX Aspiration Catheter because it may damage the device.
Potential Adverse Events
Possible complications include, but are not limited to, the following:
allergic reaction and anaphylaxis from contrast media; acute occlusion; air embolism; arteriovenous fistula; death; device malfunction; distal embolization; emboli; false aneurysm formation; hematoma or hemorrhage at access site; inability to completely remove thrombus; infection; hemorrhage; ischemia; kidney damage from contrast media; neurological deficits including stroke; vessel spasm, thrombosis, dissection, or perforation; intimal disruption; myocardial infarction; emergent surgery; fibrillation; hypotension; respiratory failure; peripheral thromboembolic events.
Penumbra ENGINE – Indication For Use
The Penumbra ENGINE is indicated as a vacuum source for Penumbra Aspiration Systems.
Contraindications
There are no contraindications.
Warnings/Precautions
• The canister is intended for single use only. Do not reuse. Reuse may result in canister cracking or vacuum filter blockages, which may result in the inability to aspirate.
• Do not block bottom or back air vents. Unit may overheat and shut off or fail to restart if run for extended periods without airflow.
• To avoid risk of electrical shock, this equipment must only be connected to a supply mains with protective earth.
• Do not position the Penumbra ENGINE so that it is difficult to remove the power cord. The means of mains disconnect is to remove the power cord.
• Only use replacement fuse with correct rating (see Table 1 for fuse rating).
• Remove and service the Penumbra ENGINE if liquids or solids have been drawn into the Penumbra ENGINE.
• Do not use in the presence of a flammable anesthetic mixture with air or nitrous oxide.
• Do not use in an oxygen rich environment.
• To prevent fire or shock hazard, use a replacement power cord of equal rating.
• Do not re-infuse blood or fluid from the canister back into the patient.
• Do not use petroleum base compounds, acids, caustics, or chlorinated solvents to clean or lubricate any parts. It will reduce service life of the Penumbra ENGINE. Use only waterbased solvents for cleaning.
• Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
• Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Penumbra ENGINE. Otherwise, this could result in degradation of the performance of this equipment.
• Common emitters (such as RFID emitters, security systems, diathermy equipment, and portable transmitters) should not be used in close proximity to the Penumbra ENGINE as they can interfere with and result in degradation of the performance of the equipment.
• Equipment is not safe for MR use.
• No modification of this equipment is allowed.