Manufacturer > Boston Scientific > Devices > Jetstream™ Atherectomy System
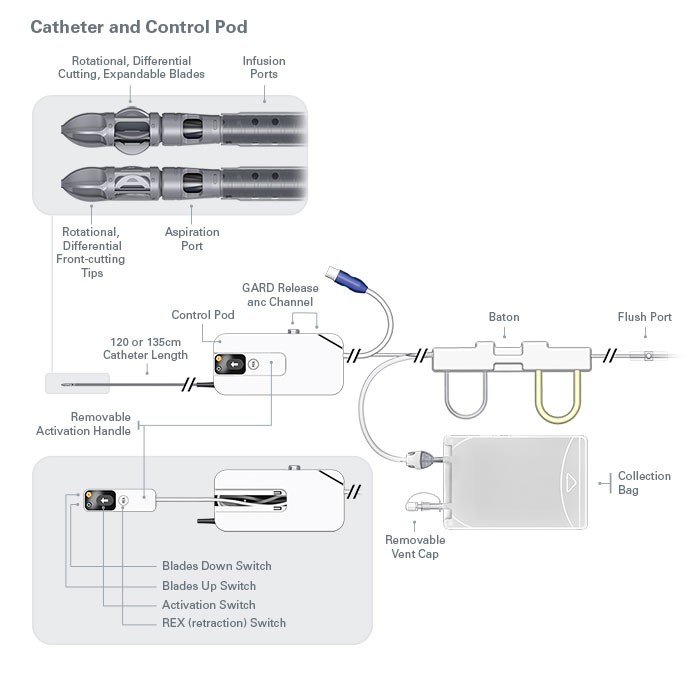
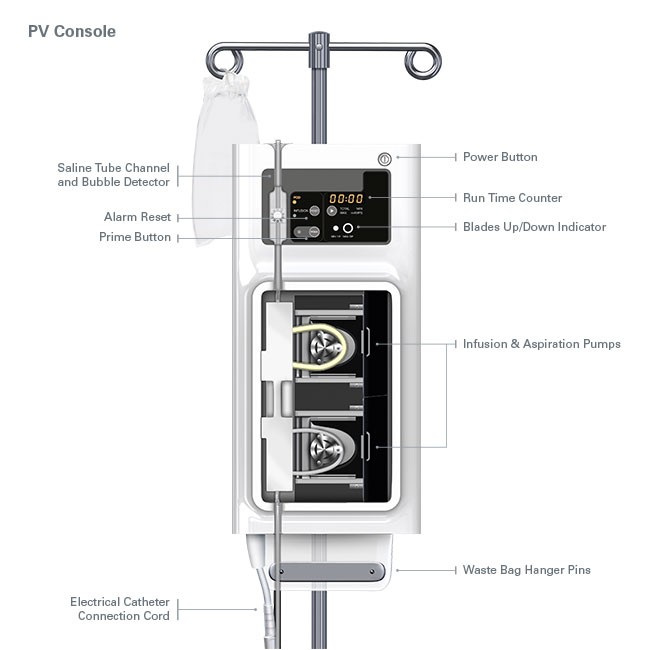
Jetstream™ Atherectomy System
Jetstream is engineered to predictably treat multiple morphologies, such as calcium, plaque or thrombus, commonly found in total occlusions. Jetsream atherectomy system has active aspiration, which minimizes the risk of distal embolization.
Catheter INTENDED USE/INDICATIONS FOR USE
The JETSTREAM System is intended for use in atherectomy of the peripheral vasculature and to break apart and remove thrombus from upper and lower extremity peripheral arteries. It is not intended for use in coronary, carotid, iliac or renal vasculature.
Console INTENDED USE/INDICATIONS FOR USE
The PVCN100 Console is designed for use only with the JETSTREAM Catheter and Control Pod.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×