Manufacturer > BD > Devices > LifeStent™ Solo™ Vascular Stent System
LifeStent™ Solo™ Vascular Stent System
Device-Type
Self-Expanding Stents
Manufacturer
BD
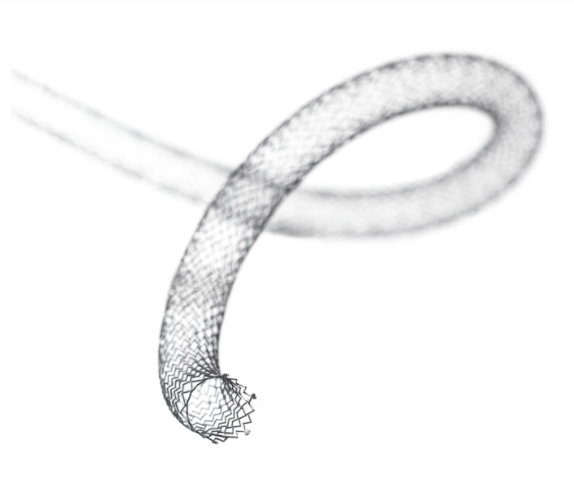
The LifeStent™ Solo™ Vascular Stent is the first commercially available 200 mm stent in the U.S. and potentially enables a single-stent solution. The LifeStent™ Solo™ Vascular Stent has sustained effectiveness up to three years in longer lesions. Furthermore, its enhanced delivery system is designed to reduce delivery system-dependent stent compression or elongation and enhance deployment accuracy. The LifeStent™ Vascular Stent Systems, in varying sizes, have been studied in more than ten clinical trials in the United States and globally.
The LifeStent™ Solo™ Vascular Stent is a peripheral stent intended to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions up to 240mm in length in the native superficial femoral artery (SFA) and popliteal artery with reference vessel diameters ranging from 4.0 – 6.5mm. The LifeStent™ Vascular Stent is the only FDA-approved stent for the SFA and full popliteal artery. The LifeStent™ Solo™ Vascular Stent is available in 6 mm and 7 mm diameters and 200 mm in length.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers