Manufacturer > Medtronic > Devices > Mo.Ma Ultra Proximal Cerebral Protection Device
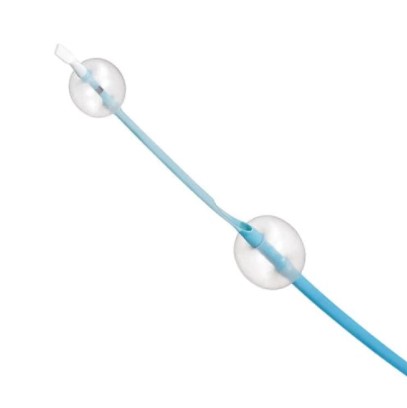
Mo.Ma Ultra Proximal Cerebral Protection Device
Device-Type
Embolic Protection
Manufacturer
Medtronic
Use the Mo.Ma™ Ultra proximal cerebral protection device to contain and remove all sizes of debris that can dislodge during interventional procedures in the carotid arteries. The Mo.Ma Ultra device with double-occlusion balloon system allows for proximal embolic protection to be established prior to crossing a carotid lesion.
The Mo.Ma™ Ultra proximal cerebral protection device is indicated as an embolic protection system to contain and remove embolic material (thrombus/debris) while performing angioplasty and stenting procedures involving lesions of the internal carotid artery and/or the carotid bifurcation.
The reference diameter of the external carotid artery should be between 3-6 mm and the reference diameter of the common carotid artery should be between 5-13 mm.
Features and Benefits
Safety informations
Device Documents
Questions & Answers