Manufacturer > Merit Medical > Devices > ONE Snare® Endovascular Snare System
ONE Snare® Endovascular Snare System
Device-Type
Snares
Manufacturer
Merit Medical
DESCRIPTION:
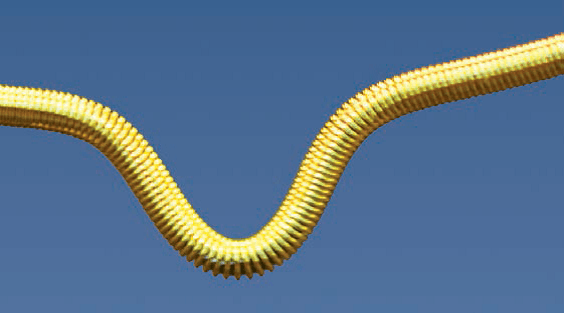
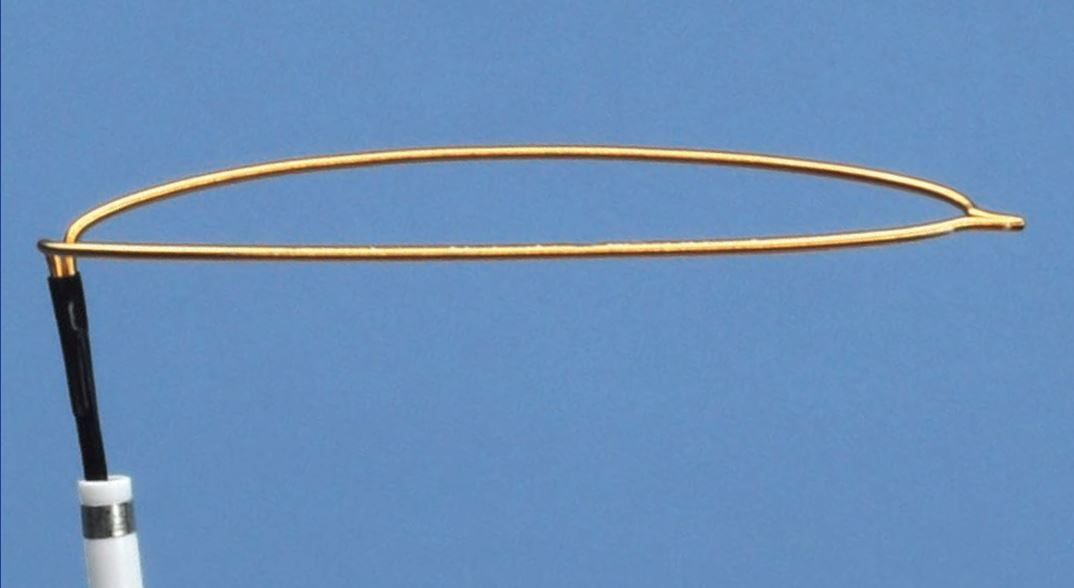
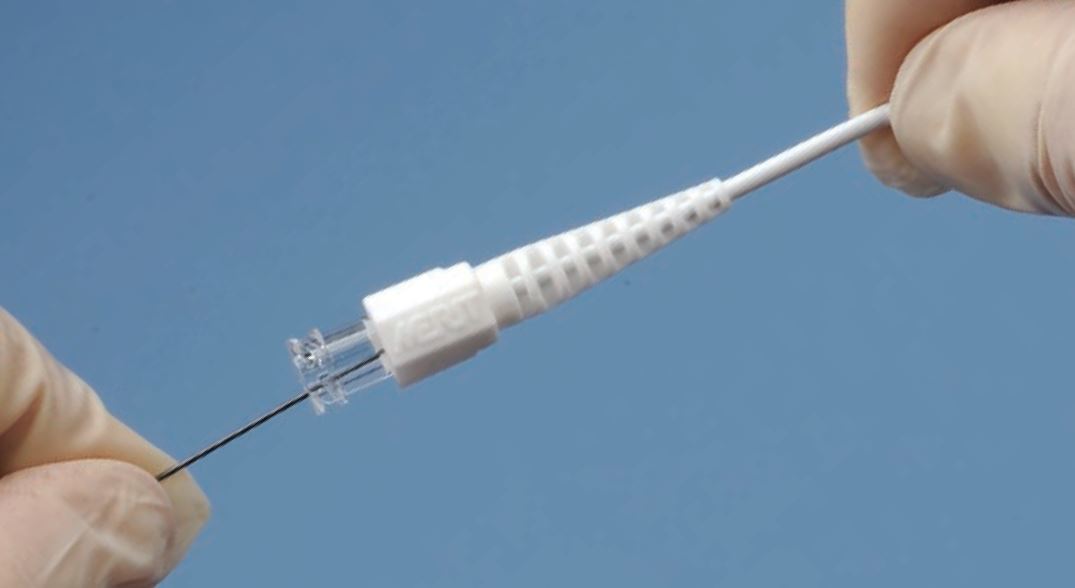
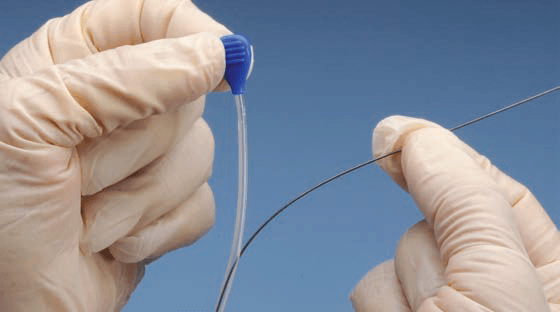
The ONE Snare™ endovascular snare system consists of the snare, snare catheter, insertion tool and torque. The snare is constructed of nitinol cable and gold plated tungsten loop. The pre-formed snare loop can be introduced through catheters without risk of snare deformation because of the snare’s super-elastic construction. The snare catheter is constructed of polyether block amide and contains a platinum/iridium radiopaque marker band.
INDICATION FOR USE:
The ONE Snare™ endovascular snare system is intended for use in the coronary and peripheral vascular system or hollow viscous to retrieve and manipulate foreign objects. Retrieval and manipulation procedures include indwelling venous catheter repositioning, indwelling venous catheter fibrin sheath stripping, and central venous access venipuncture procedure assistance.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers