Device-Type
Intravascular Ultrasound
Targated Speciality
Cardiovascular
Manufacturer
Boston Scientific
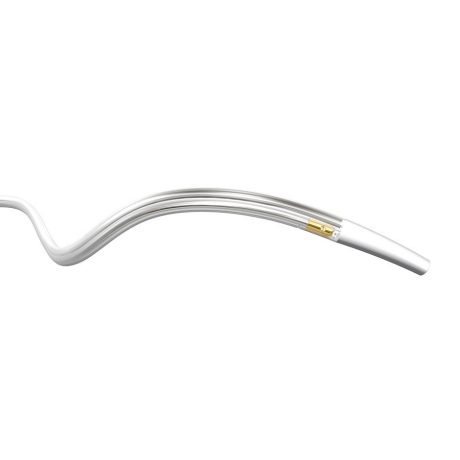
The OptiCross 35 peripheral imaging catheter is the latest addition to Boston Scientific’s IVUS portfolio. The catheter’s unique design provides exceptional image quality leading to more confident detection and treatment of venous disease.
Features and Benefits
Recommended Compatible Devices
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×