CONTRAINDICATIONS:
If other interventional devices are used in conjunction with the Ostial PRO Stent Positioning System, refer to specific manufacturer’s product labeling for intended use, contraindications and potential complications associated with that device.
PRECAUTIONS:
• For single use only. Do not resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure, which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient.
• Federal law (U.S.A.) restricts this device to sale by or on the order of a physician.
• Device is sterile if the package is dry, unopened and undamaged. Do not use if sterile barrier is damaged.
• The device must be used prior to the expiration date.
• Discard device if mishandling has caused possible damage or contamination.
• This device should be stored in a clean, dry location at room temperature.
• This device has been sterilized with ethylene oxide gas.
• Inspect the Ostial PRO Stent Positioning System prior to use for any bends or kinks. Any Ostial PRO Stent Positioning System damage may decrease the desired performance characteristics.
• These Instructions For Use are designed to serve only as a general guideline. They are not intended to supersede institutional protocols or professional clinical judgment concerning patient care.
WARNING:
If extended procedure is required (greater than 30 minutes), it is recommended to not load the Ostial PRO Stent Positioning System into the guiding catheter until it is needed to aid in the placement of the stent. For these procedures, utilize the “Front Loading” steps for the Ostial PRO Stent Positioning System introduction into the guiding catheter.
b. Front Loading
i. Remove the blue introducer tube from the plastic shipping hoop.
ii. Load the Ostial PRO Stent Positioning System into the introducer tube by inserting the proximal (yellow) end of the Ostial PRO Stent Positioning System wire into the blue introducer.
iii. Pull the Ostial PRO wire through the introducer until the Ostial PRO Stent Positioning System body is positioned inside the diameter of the blue introducer.
iv. If a guide wire is engaged in the guiding catheter, slide the blue introducer tube over the proximal end of the guide wire.
v. Insert the introducer tube and Ostial PRO Stent Positioning System together into the proximal end of the Tuohy-Borst adaptor.
vi. Slide the blue introducer tube through the Tuohy-Borst until it contacts the proximal end of the hub on the guiding catheter. Blue introducer will stop.
vii. At this time, push the Ostial PRO Stent Positioning System forward until it passes out the distal end of the blue introducer tube. The Ostial PRO Stent Positioning System will now be in the guiding catheter.
viii. The Ostial PRO Stent Positioning System can now be slid distally until it is proximal to the primary curve of the guiding catheter.
5. Advance a 0.014”-0.025” (.35 mm - .64 mm) guide wire into the Tuohy-Borst through the guiding catheter crossing the ostial lesion and positioned in the distal portion of the target vessel.
6. Advance the stent catheter system through the Tuohy-Borst over the guide wire until the stent catheter system is distal to the diseased area to be treated.
7. Disengage the guiding catheter from the ostium of the vessel, pulling the guide tip back approximately 3-5 mm and confirm that the guiding catheter is in the aorta using a small test injection of contrast.
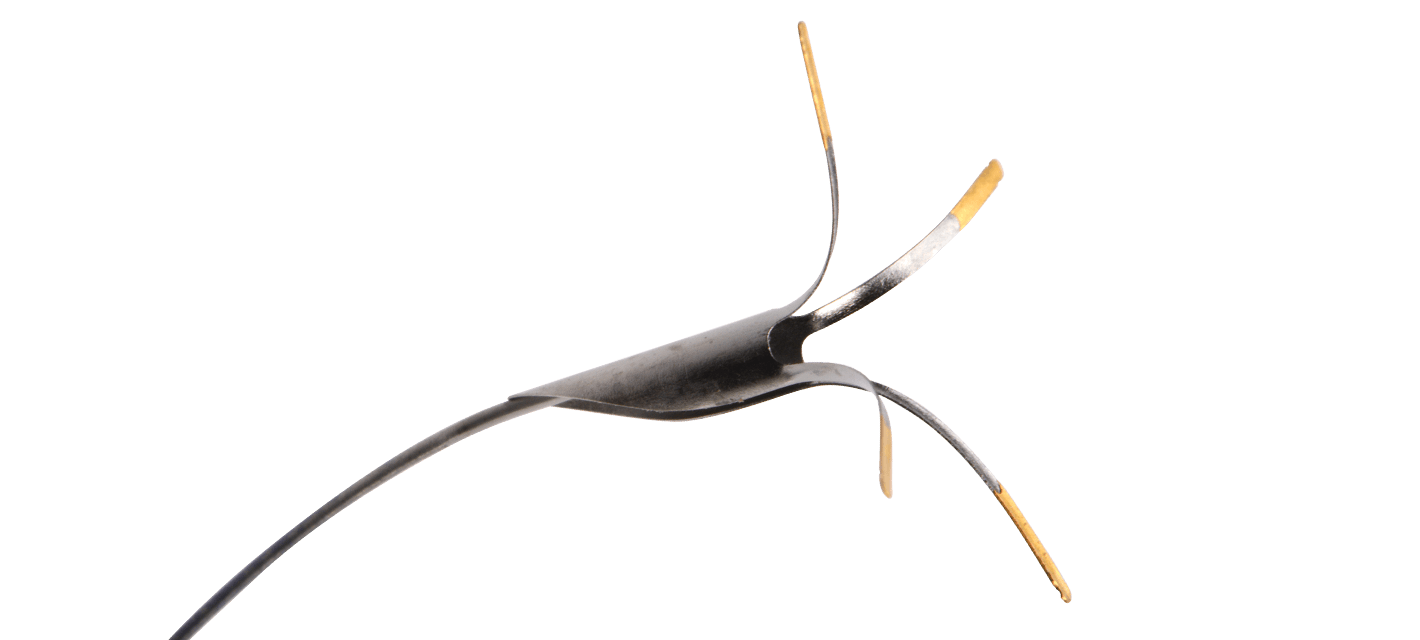
8. While holding the stent catheter system and guide wire in a stable position, gently push the Ostial PRO Stent Positioning System forward until the gold plated distal feet expand into an open position, using fluoroscopic guidance.
9. Push the guiding catheter and Ostial PRO Stent Positioning System, together as one single unit, forward until the gold-plated feet are clearly in contact with the aortic wall. Inject contrast medium to confirm the guiding catheter, Ostial PRO Stent Positioning System, and lesion positions (“Power Position”).