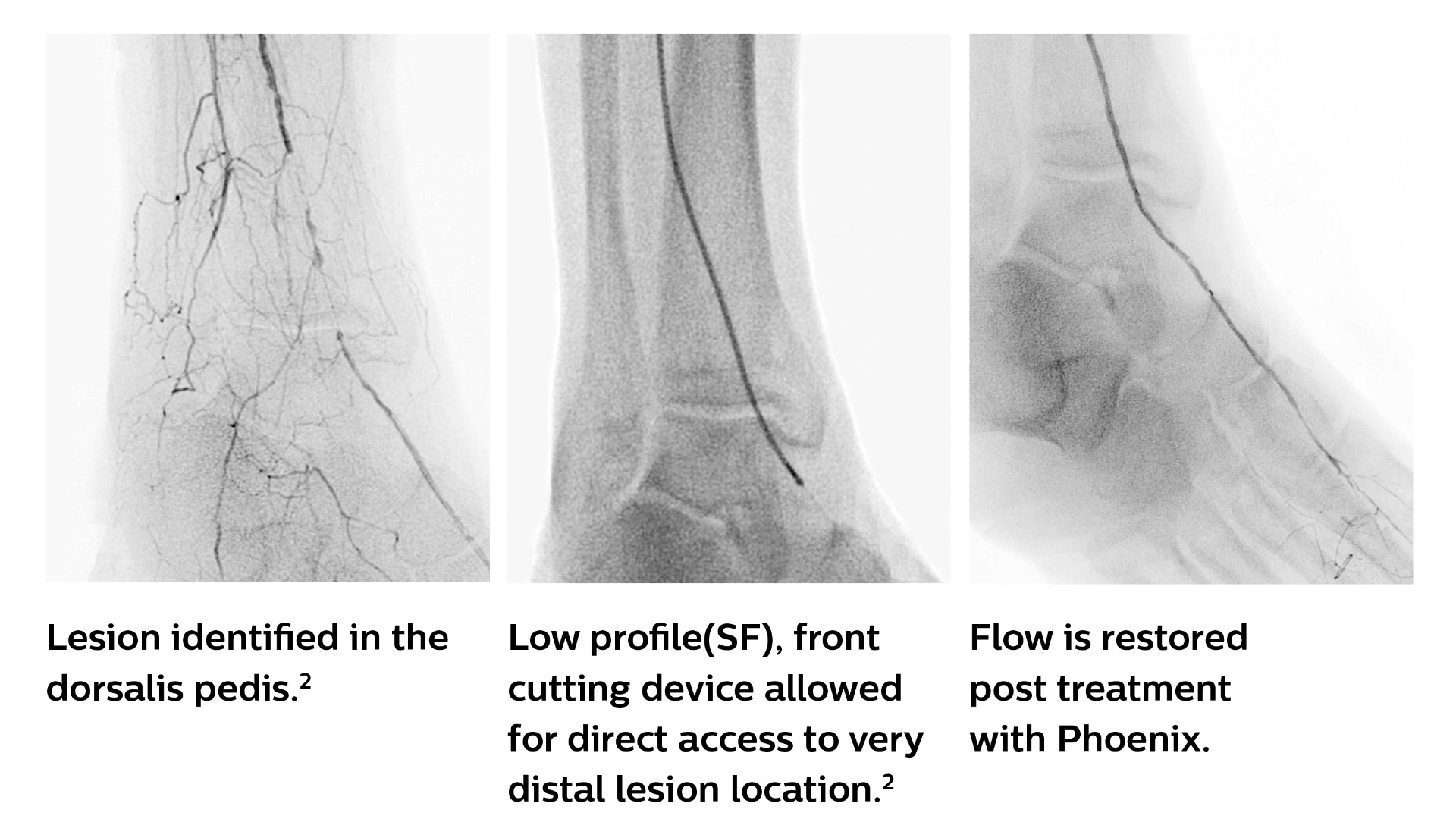
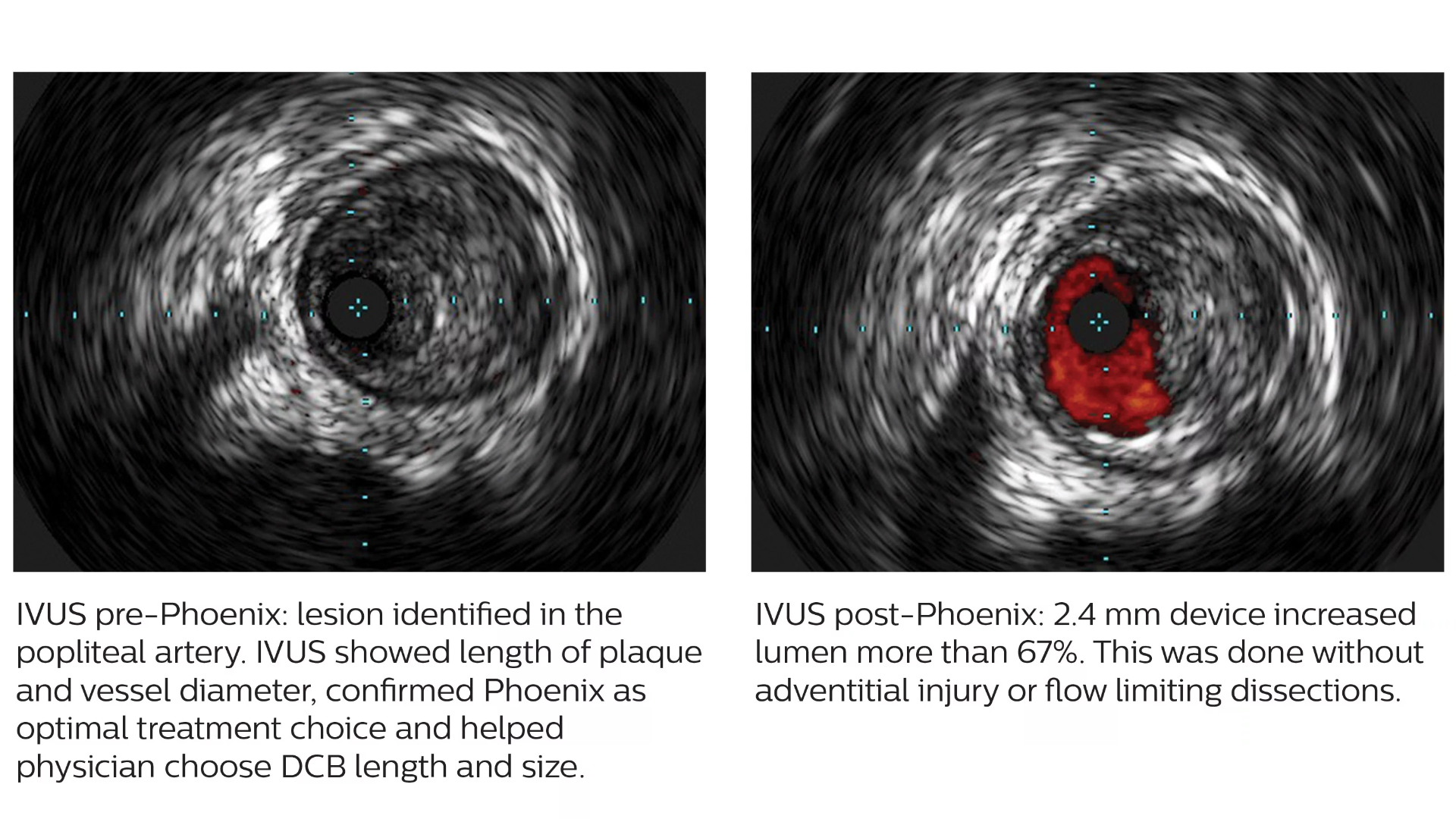
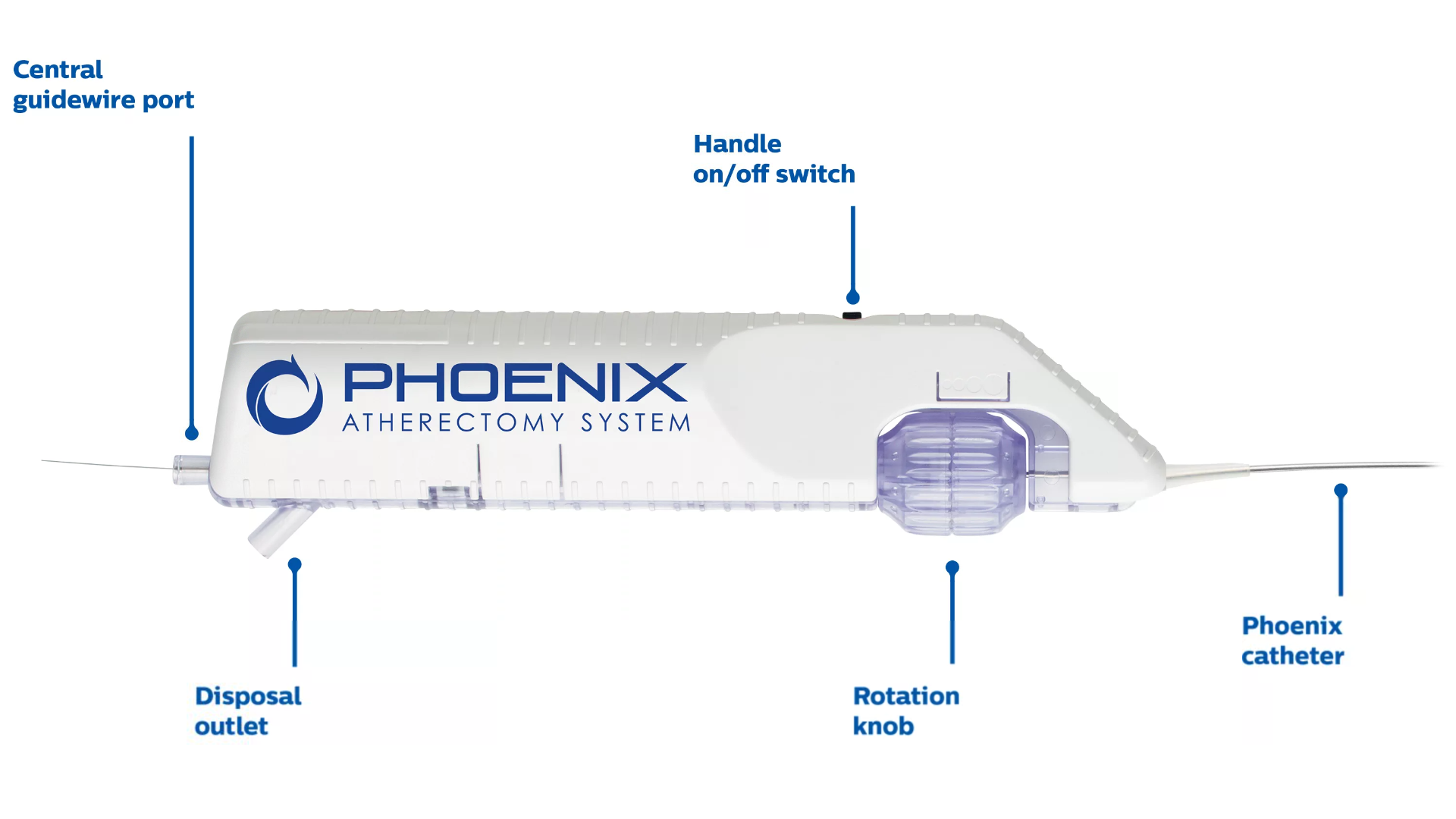
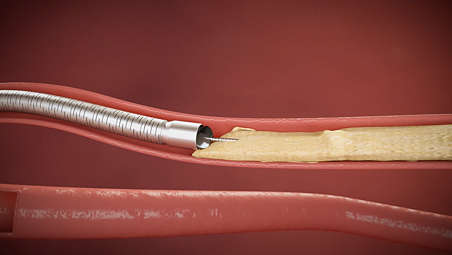
The Phoenix atherectomy system combines the benefits of existing atherectomy systems to deliver a unique, hybrid atherectomy option to help physicians tailor the treatment approach for each patient. It cuts, captures, and clears diseased tissue with one insertion. Phoenix treats a broad range of tissue types, from soft plaque to calcified arteries, and can be used for lesions above and below the knee.
Features and Benefits
Device Documents
Questions & Answers
×