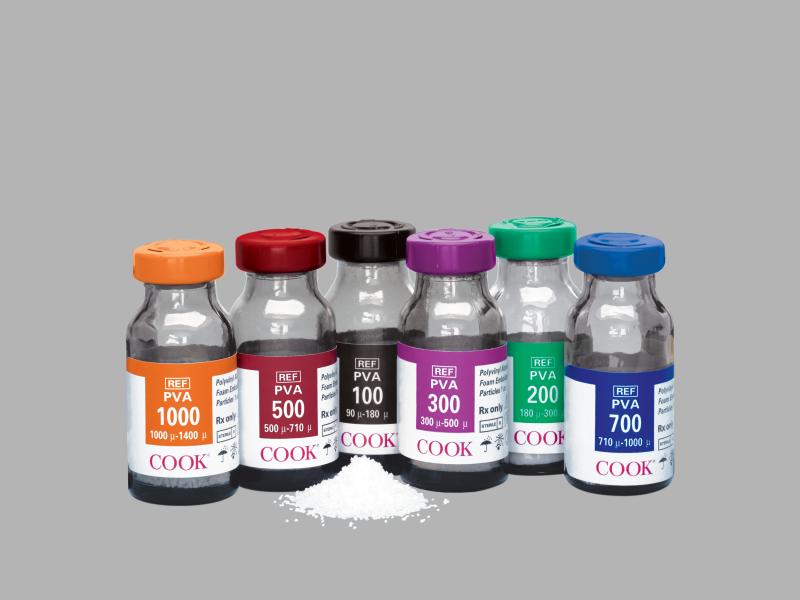
Manufacturer > Cook Medical > Devices > PVA Foam Embolization Particles
PVA Foam Embolization Particles
Device-Type
Embolic Particles/Beads
Manufacturer
Cook Medical
INTENDED USE :-
Polyvinyl Alcohol Foam Embolization Particles are intended for embolization of the blood supply to hypervascular tumors and arteriovenous malformations, including use in intracranial embolization. The product is intended for use by physicians trained and experienced in embolization procedures in the targeted area. Standard techniques for embolization procedures should be employed.
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×