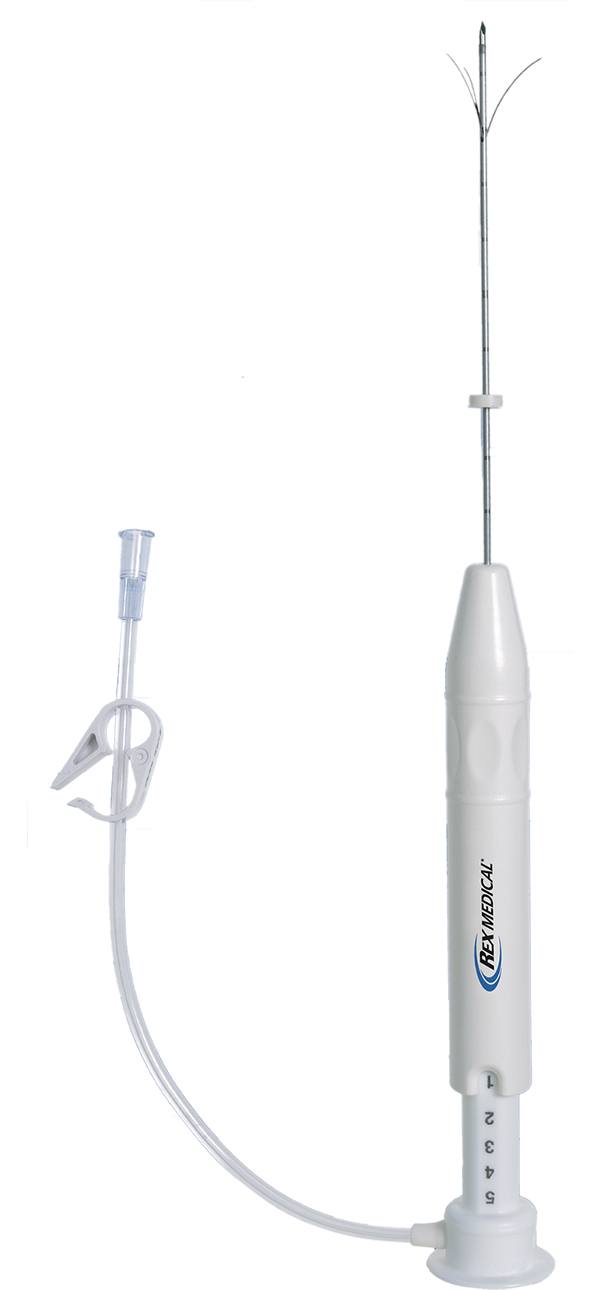
PRECAUTIONS
1. Always use standard sterile, aseptic technique.
2. Always flush Quadra-Fuse*/Quadra-Fuse* ST needle with sterile heparinized saline solution prior to use.
3. Do not use the device if there is evidence of mechanical damage or leaking.
4. Fill (prime) the device prior to use to minimize potential for cannula obstruction. Once the device is properly primed, be sure to close the extension tubing clamp.
5. Excessive force should never be used to place needle or prongs.
6. Excessive force should not be used to infuse or flush obstructed lumen. Do not use a larger syringe than 10m1 (cc).
7. Be sure to confirm proper Quadra-Fuset/Quadra-Fuse* ST needle position with proper imaging guidance (i.e. CT, US, Fluoroscopy).
8. Before needle cannula insertion (use), be sure to retract plunger completely. Needle prongs should be completely contained inside cannula prior to insertion.
9. As necessary, clean the needle array between deployments by rinsing the needle array in sterile heparinized saline solution or by gently wiping the prongs to remove excess tissue. Accumulation of excess tissue on the prongs may make array retraction difficult.
10. It is recommended that only luer lock (threaded) accessories and components be used with the Quadra-Fuse*/Quadra-Fuse* ST needle luer connector. Repeated over tightening of syringes will reduce connector life and could lead to potential connector failure.
11. Federal (USA) law restricts this device to be sale by or under the direction of physician.
WARNINGS
1. Infusion through Quadra-Fuse*/Quadra-Fuse* ST needle luer connector can only be initiated after all air is removed from the device.
2. Due to risk of exposure to MV (Human Immunodeficiency Virus) or other blood borne pathogens, health care workers should routinely use universal blood and body-fluid precautions in the care of all patients. Sterile technique must be strictly adhered to during any handling of the device.