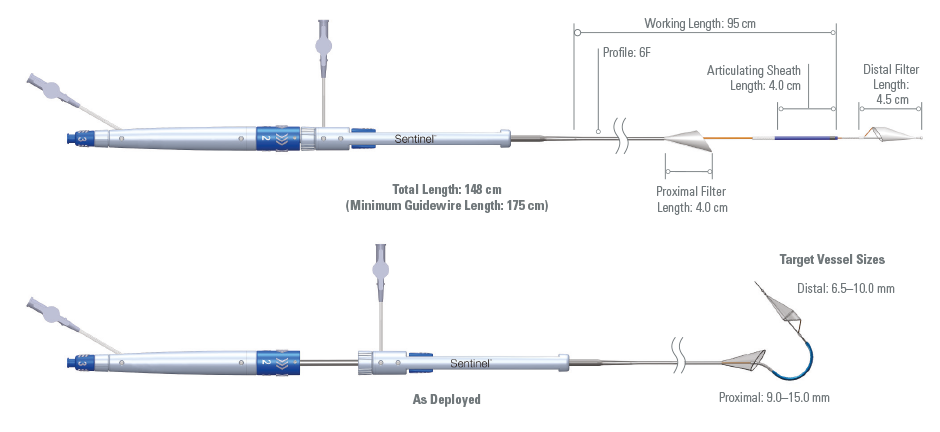
CONTRAINDICATIONS
• Do not use in patients for whom anticoagulant and antiplatelet therapy is contraindicated.
• Do not use in patients with a known hypersensitivity to nickel-titanium.
• Do not use in vessels with excessive tortuosity.
• Do not use in patients with uncorrected bleeding disorders.
• Do not use in patients with compromised blood flow to the right upper extremity.
• Do not use in patients who have arterial stenosis >70% in either the left common carotid artery or the brachiocephalic artery.
• Do not use in patients whose brachiocephalic or left carotid artery reveals significant stenosis, ectasia, dissection, or aneurysm at the aortic ostium or within 3 cm of the aortic ostium.
WARNINGS
• The appropriate antiplatelet/anticoagulation therapy should be administered pre- and post-procedure in accordance with standard medical practice.
• It is recommended that the patency of the right radial or brachial artery be assessed prior to the introduction of the SENTINEL System.
• It is recommended that the patient be tested for occlusion of the radial or brachial artery prior to device introduction.
• Do not use the device in left radial or left brachial access.
• Do not use the SENTINEL System to deliver any type of fluid to the patient e.g. contrast media, heparinized saline, etc. due to risk of air embolization and comprise to device performance. Excessive movement of filters may lead to embolization of debris, vessel and/or device damage.
• Do not deploy the filters within a previously repaired artery, an artery that has been used for dialysis purposes, or an AV fistula.
• Indwell time of the SENTINEL System is not to exceed 90 minutes as occlusion could occur, resulting in slow or no flow.
• Do not undersize or oversize the filters in relation to the selected vessel diameter. This may result in inadequate vessel wall apposition or incomplete deployment of the filters.
PRECAUTIONS
• Do not forcefully bend or reshape the Articulating Sheath of the SENTINEL System.
• Use of TAVR delivery systems other than those designed to cross the aortic arch with a valve frame in a sheathed or crimped configuration may result in device interference or entanglement.