Manufacturer > Argon Medical Devices > Devices > Single-loop and Triple-loop Retrieval Kit
Single-loop and Triple-loop Retrieval Kit
Device-Type
Snares
Manufacturer
Argon Medical Devices
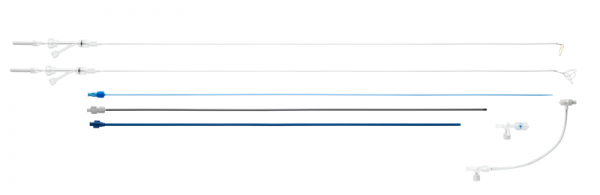
Single-Loop Snare Retrieval Kits and Triple-Loop Snare Retrieval Kits consist of a 9F inner sheath, 11F outer sheath, 8F dilator, hemostasis valve with sideport, high pressure stopcock, 20mm single-loop snare (fully expanded) or 30mm triple-loop snare (fully expanded), 7F snare catheter, Tuohy-Borst Y-port adapter, and torque handle. The snares have radiopaque loops and are preloaded in the snare catheter. The snare catheter, inner sheath, and outer sheath have a radiopaque marker band at the distal tip for enhanced fluoroscopic visualization. The maximum recommended infusion rate is 15mL/sec for power injection of undiluted contrast at body temperature through the dilator, outer sheath and high pressure stopcock. This product is not made with natural rubber latex.
With a rigid sheath, enhanced visualization, hemostatic control and two snare options, Argon’s Single-Loop and Triple-Loop Retrieval Kits are designed with the rigor of the IVC filter retrieval procedure in mind.
Indication for Use
Single-Loop Snare Retrieval Kits and Triple-Loop Snare Retrieval Kits are intended for the percutaneous removal of retrievable inferior vena cava (IVC) filters that are no longer medically required, via jugular approach.
Each kit includes:
- 11F (ID) x 60cm Outer Sheath
- 9F (ID) x 64cm Inner Sheath
- 8F (OD) x 75cm Dilator
- Hemostasis Valve with Sideport
- 3-Way High Pressure Stopcock
- (Triple Loop) 7F (OD) x 76cm Snare Catheter with Tuohy-Borst Y-Port Adapter, 30mm x 93cm Triple-Loop Snare with Torque Handle
Or
- 11F (ID) x 60cm Outer Sheath
- 9F (ID) x 64cm Inner Sheath
- 8F (OD) x 75cm Dilator
- Hemostasis Valve with Sideport
- 3-Way High Pressure Stopcock
- (Single Loop) 7F (OD) x 76cm Snare Catheter with Tuohy-Borst Y-Port Adapter, 20mm x 93cm Single-Loop Snare with Torque Handle
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers