Manufacturer > Sirtex > Devices > SIR-Spheres® Y-90 resin microspheres
SIR-Spheres® Y-90 resin microspheres
Device-Type
Selective Internal Radiation Therapy
Manufacturer
Sirtex
SIR-Spheres® Y-90 resin microspheres are a medical device used in Selective Internal Radiation Therapy (SIRT) for liver tumors.
- SIR-Spheres Y-90 resin microspheres are a permanent implant and for single use only.
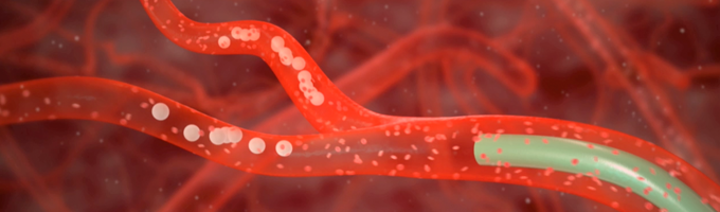
- The biocompatible resin microspheres labelled with yttrium-90 have a median diameter of 32.5 microns (range between 20 and 60 microns).
SIR-Spheres®Y-90 resin microspheres
- are directly targeting liver tumors via the hepatic artery
- minimize exposure to the remaining healthy liver tissue
Due to their relatively low density of 1.1 g/mL, SIR-Spheres Y-90 resin microspheres
- travel with the blood stream
- are taken deep into the tumor microvasculature where they become lodged.
SIR-Spheres Y-90 resin microspheres can therefore be delivered in a slow deliberate manner
- to achieve an even distribution
- to maximize the number of microspheres delivered,
- to optimize tumor coverage - even in patients with extensive disease.
Safety informations
Potential adverse events
Questions & Answers
×