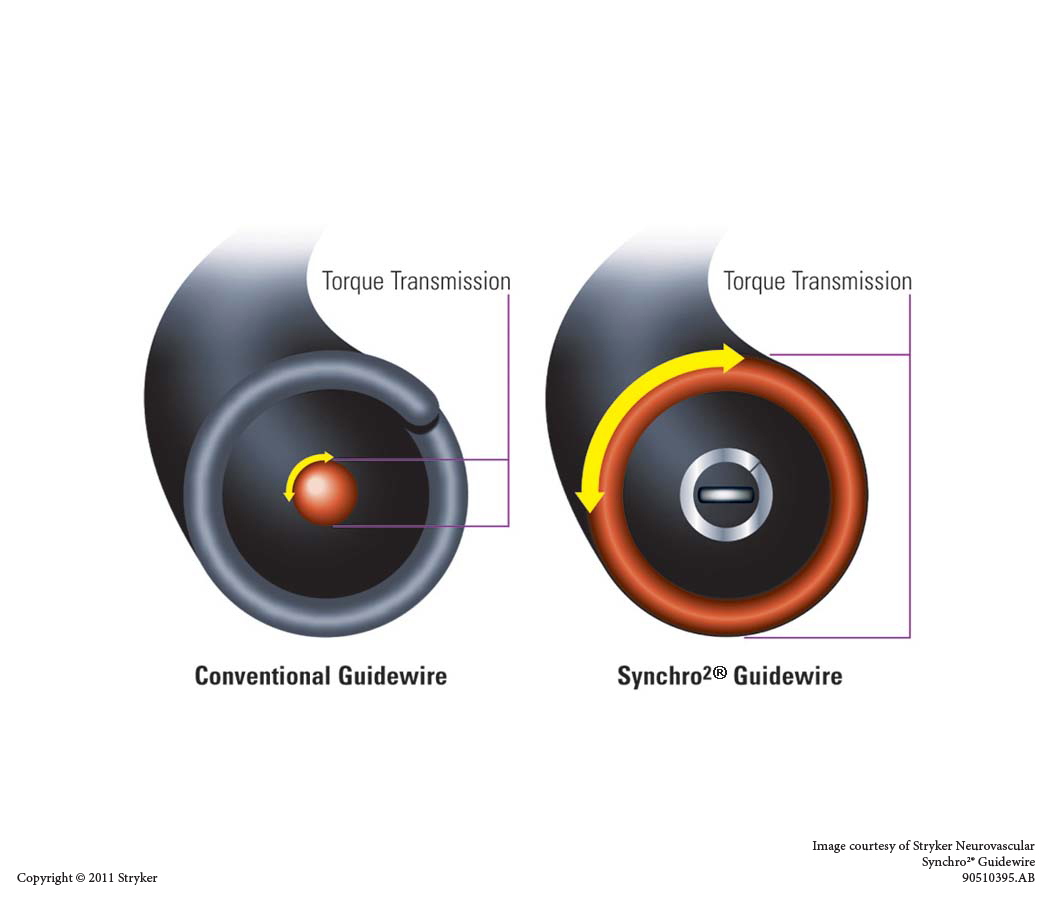
CONTRAINDICATIONS
The Synchro Neuro Guidewire series is not intended for use within the coronary vasculature. If other interventional devices are used with the Synchro Neuro Guidewire, then refer to that product labeling for intended use, contraindications and potential complications associated with the use of that interventional device.
CAUTIONS/PRECAUTIONS
•Federal law (USA) restricts this device to sale by or on the order of a physician. •Confirm the compatibility of the guidewire with the microcatheter before use. The wire should move freely within the catheter. •Securely fasten the torque device onto the wire to prevent slippage of the torque device and to avoid product damage (i.e., core wire abrasion/peeling of PTFE, etc.) •Maintain a continuous saline flush between the guiding catheter and the interventional device and between the interventional device and the guidewire during the procedure. Flushing prevents contrast crystal formation and/or clotting on the guidewire and the catheter lumen. •Verify that package integrity has not been compromised prior to use. Do not use a product after the expiration date. •Inspect the guidewire for any visible damage prior to use, and do not use a wire that is damaged. •Carefully examine all equipment for defects prior to interventional procedure. Do not use any defective equipment.
WARNINGS
•Contents supplied STERILE, using a Radiation process. Non-Pyrogenic. Do not use if sterile barrier is damaged. If damage is found, call your Stryker Neurovascular representative. •For single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of device may lead to injury, illness or death of the patient. •After use, dispose of product and packaging in accordance with hospital, administrative and/or local government policy. •As with all guidewires used in interventional procedures, complications can occur. •Before a guidewire is advanced or withdrawn, verify tip movement under fluoroscopy to prevent the possibility of vessel perforation or guidewire damage. •Do not torque a guidewire without observing corresponding movement of the distal guidewire tip; otherwise, guidewire damage, such as tip separation, and/or vessel trauma may occur. •Always advance or withdraw the guidewire slowly and carefully. Never advance, auger, withdraw, or torque a guidewire which meets resistance. Resistance may be felt and/or observed under fluoroscopy by noting any buckling or prelapse of the guide wire tip. Excessive force against resistance may result in damage to the guidewire, such as separation of the guidewire tip, damage to the interventional device and/or vessel perforation. Determine the cause of the resistance under fluoroscopy and take any necessary remedial action. The torque device and the introducer are included to aid in the use of the guidewire and are not intended to enter the patient’s body at any time. •Observe all guidewire movement in the vessel using fluoroscopy. Do not move or torque a guidewire without observing corresponding movement of the distal guidewire tip; otherwise, guidewire damage, such as tip separation, and/or vessel trauma may occur. Always advanc