Device-Type
Dissection repair device
Manufacturer
Philips
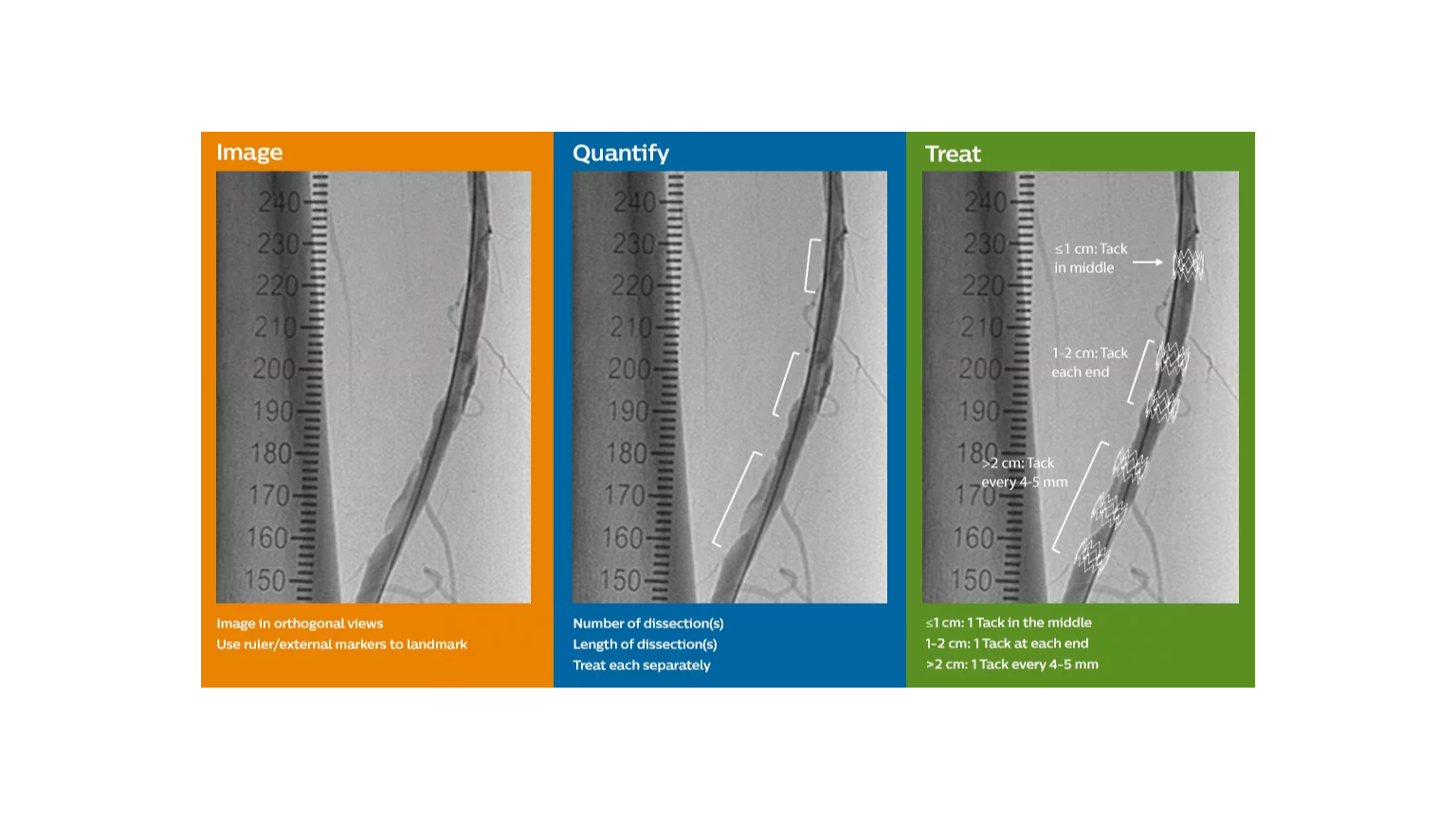
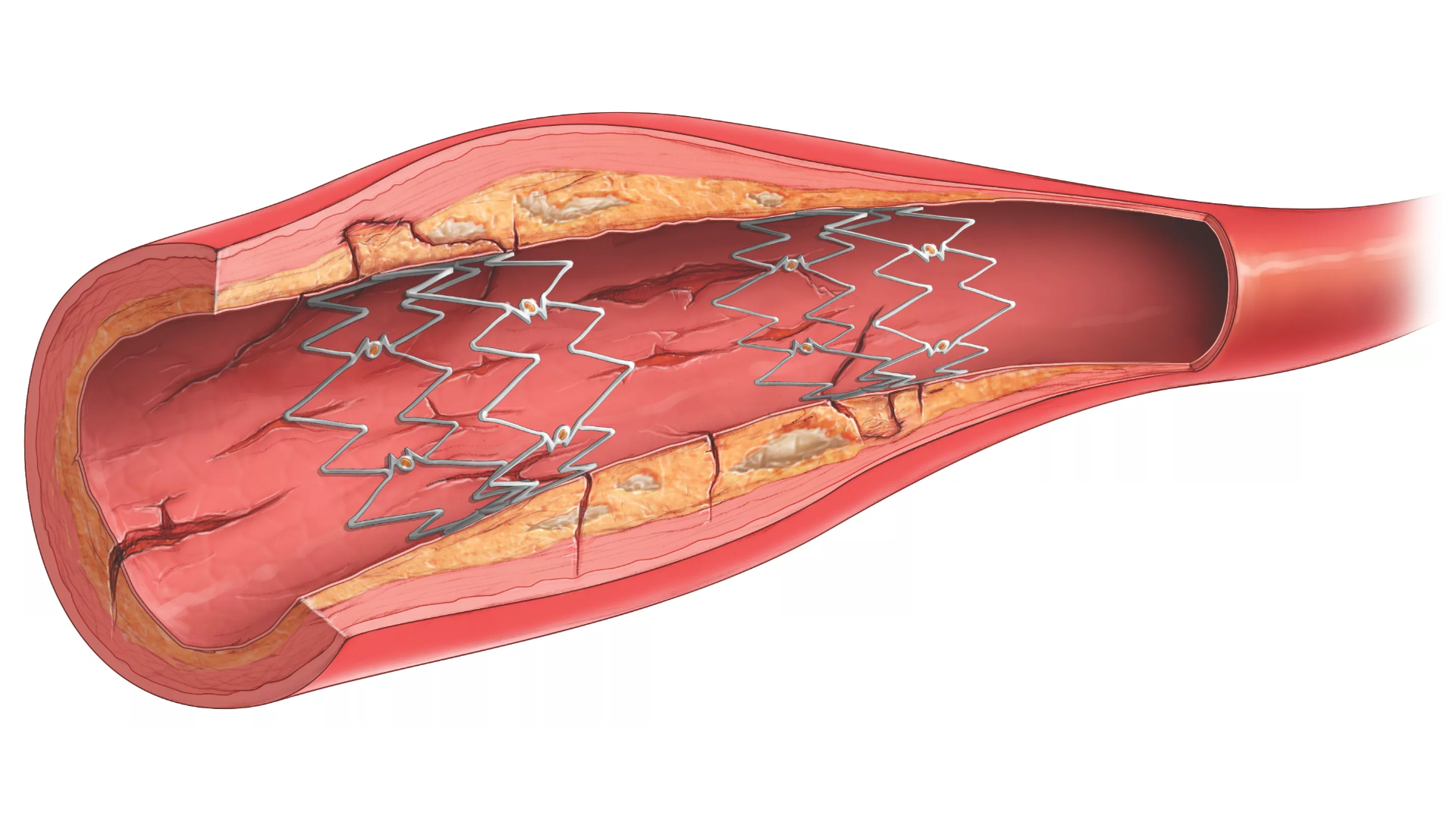
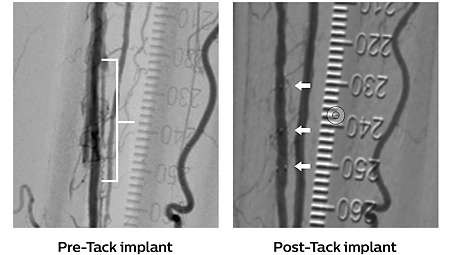
The Tack endovascular system is a first-of-its-kind, minimal-metal dissection repair device, purpose-built for precision treatment of post-PTA peripheral arterial dissections to promote healing, improve outcomes, and preserve limbs.
The Tack endovascular system is a first-of-its kind dissection repair device that is purpose-built to provide precision treatment of peripheral arterial dissections following balloon angioplasty in either above- or below-the-knee therapeutic interventions.
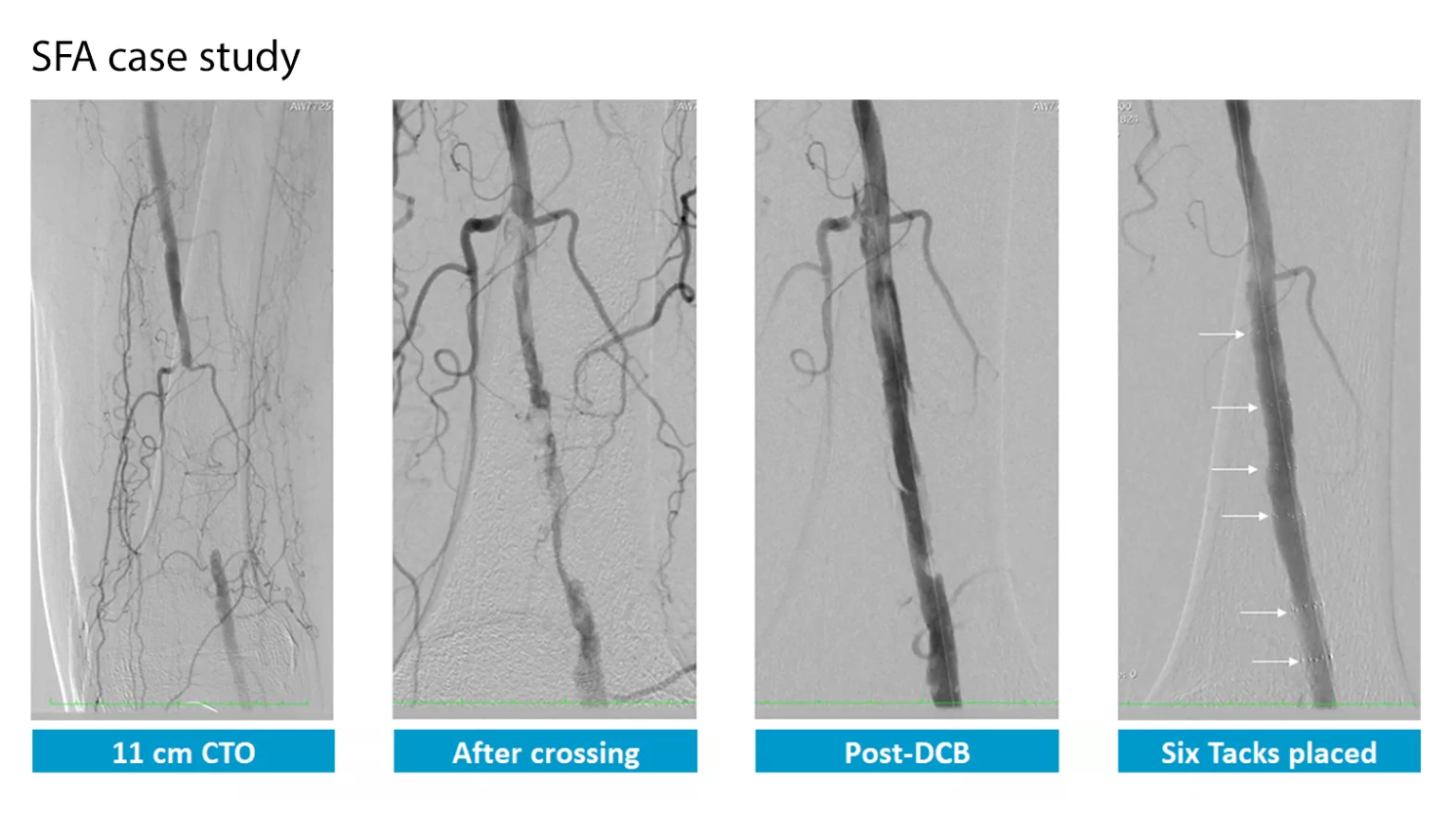
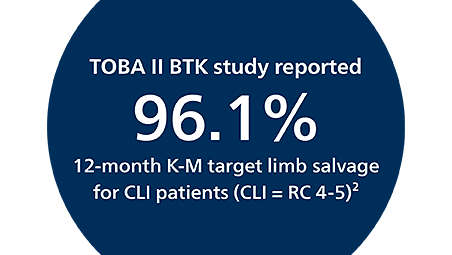
The Tack endovascular system has been rigorously studied in the Tack Optimized Balloon Angioplasty (TOBA) trials. These trials are unique in that they are the only clinical trials to investigate 100% dissected vessels. The TOBA II, TOBA III, and TOBA II BTK studies add to the large body of clinical evidence supporting the use of the Tack endovascular system, further demonstrating that post-PTA dissection repair with the Tack endovascular system improves outcomes for both POBA and DCB angioplasty for patients with PAD and CLI.
Product highlights
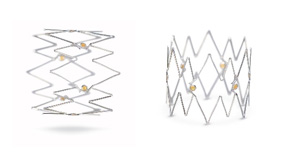
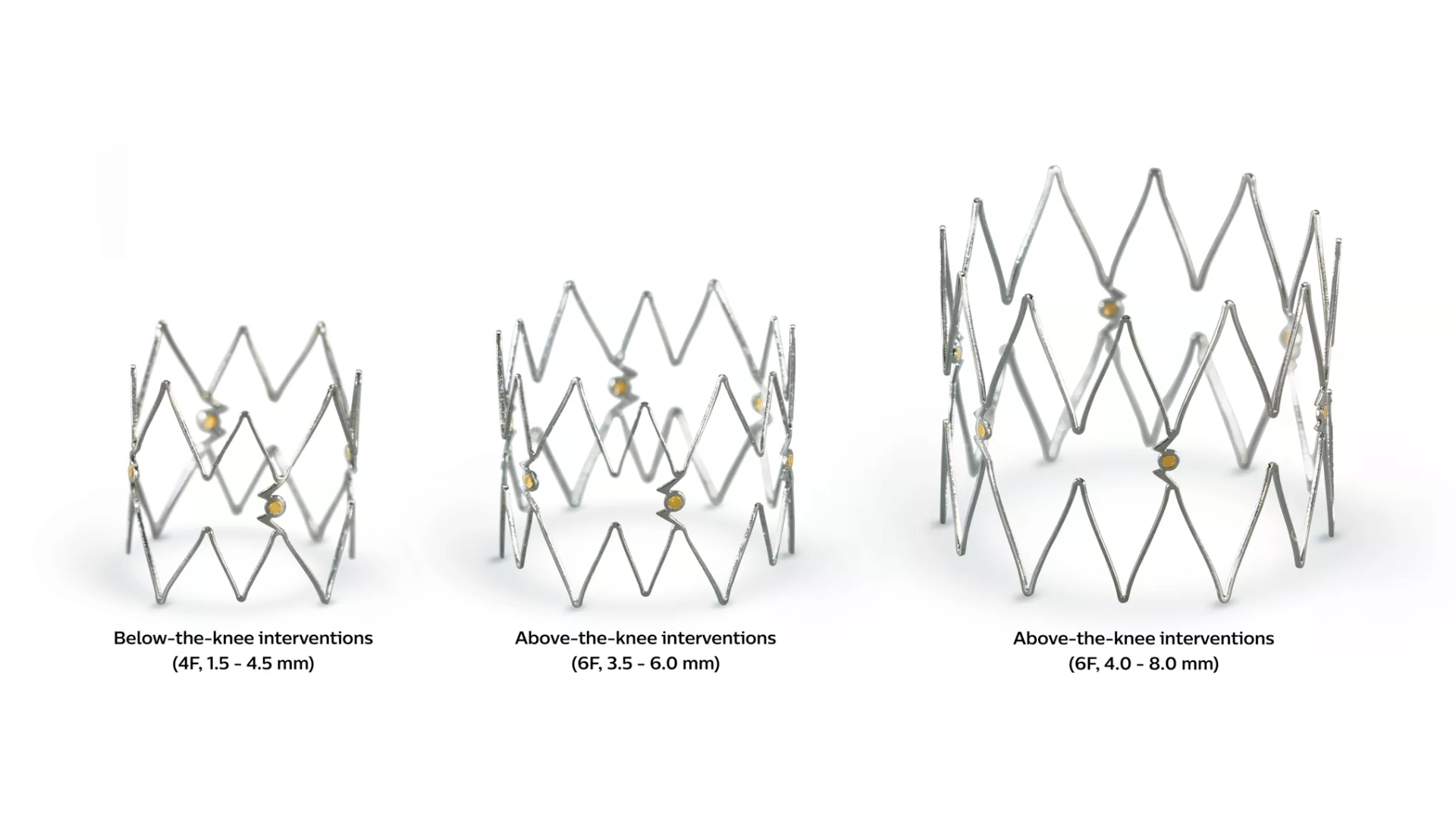
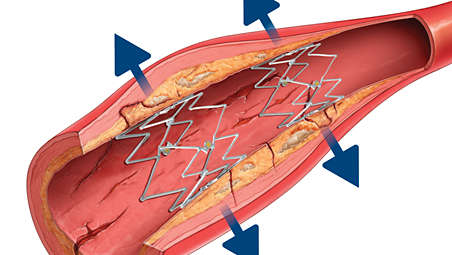
The Tack implant features adaptive sizing which allows the device to adapt to tapering anatomy while maintaining a relatively constant radial force, so that a single Tack implant can be used across a range of vessel diameters.
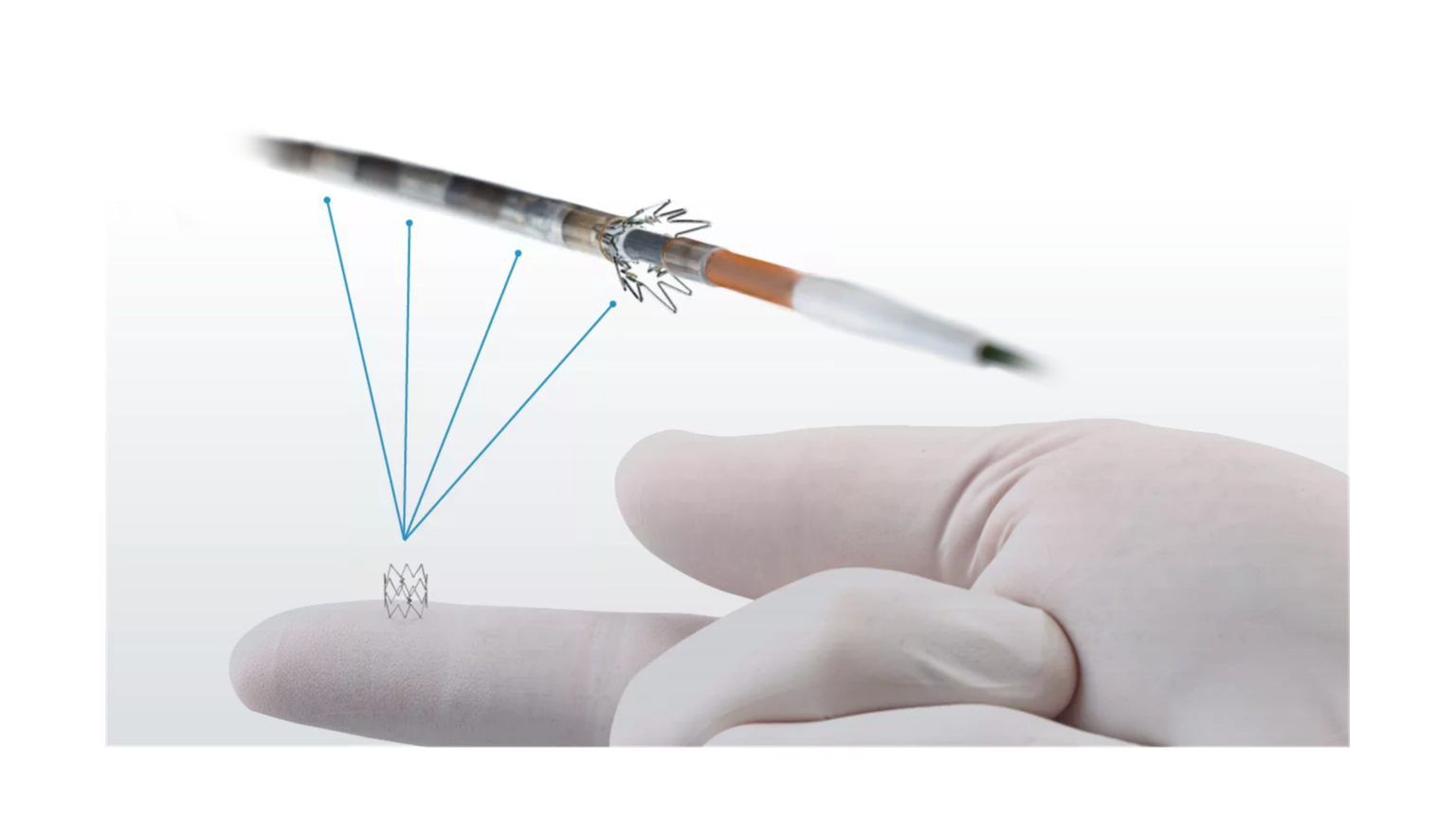
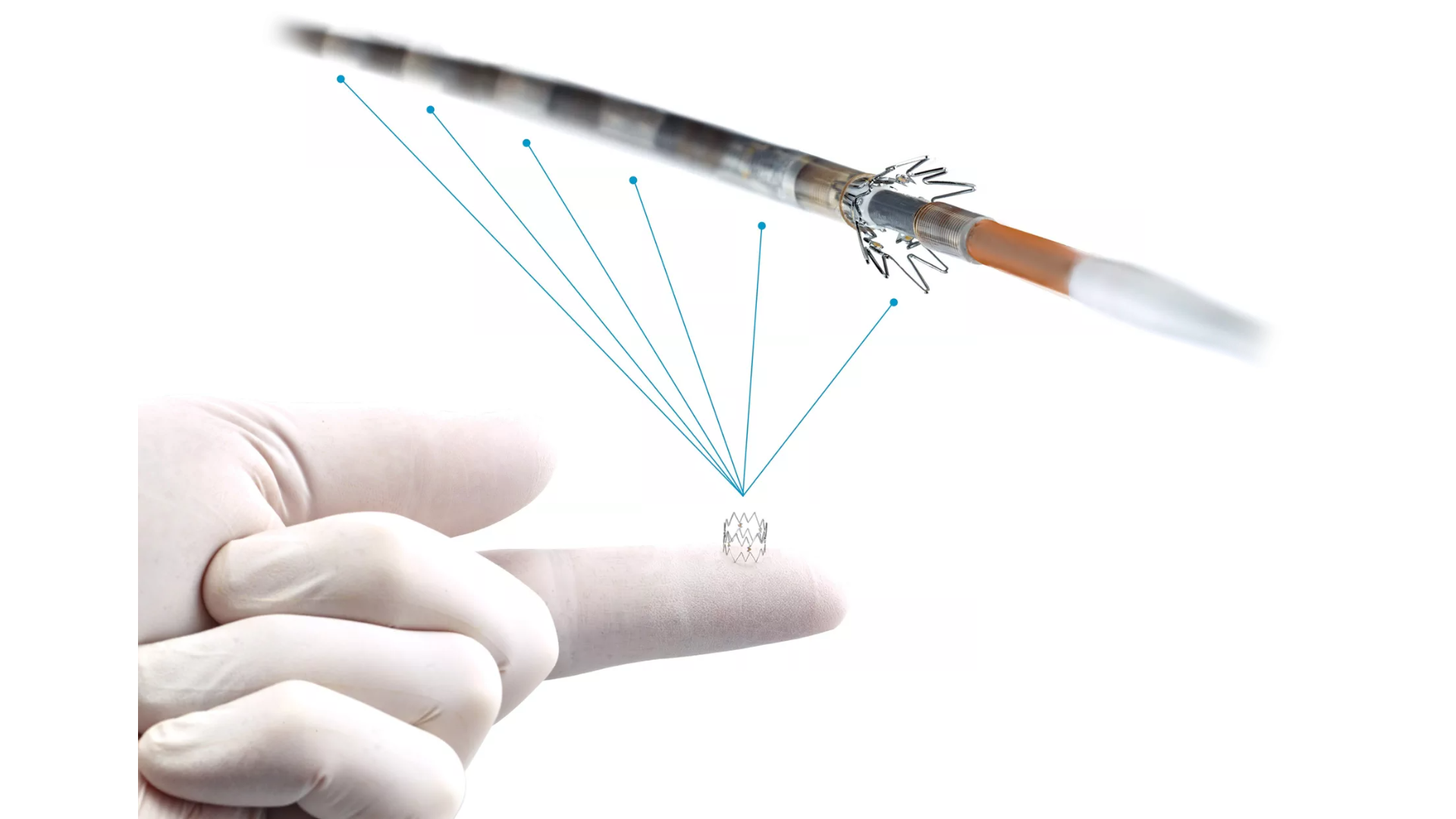
• 4F/.014” and 6F/.035” OTW delivery systems
• 120, 135, and 150 cm working lengths
• Four or six pre-loaded Tack implants
• Self-sizes to tapering vessel diameters from 1.5-4.5 mm, 3.5-6.0 mm, and 4.0-8.0 mm with a single system
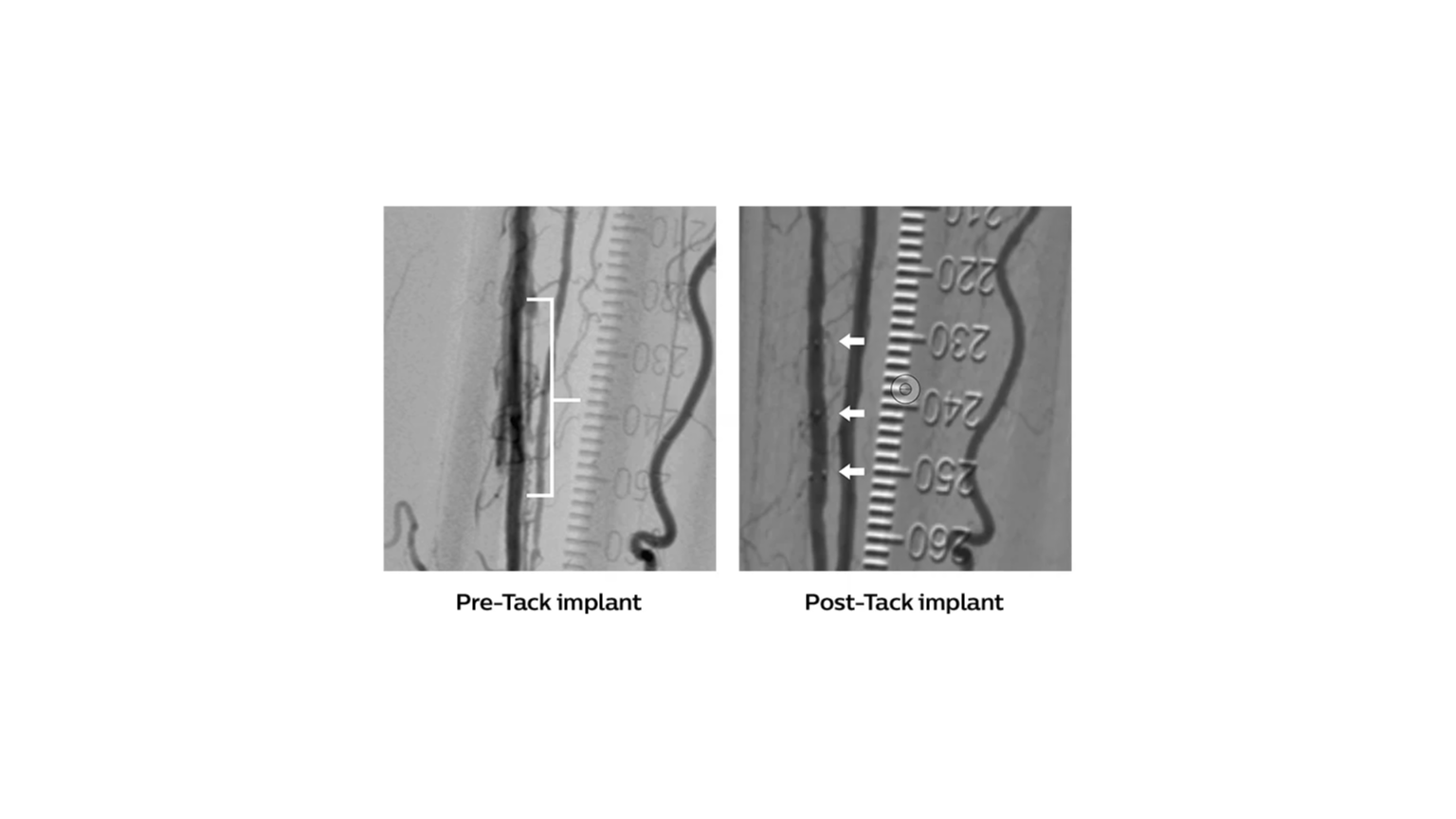
• Accurate (≤1 mm) deployment
• Nitinol with gold radiopaque markers
Features and Benefits
Safety informations
Device Documents
Questions & Answers