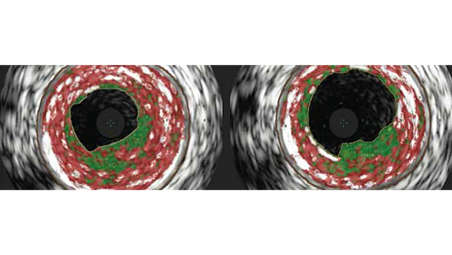
The Turbo-Elite Laser Catheter devices are indicated for use in the treatment of infrainguinal stenosis and occlusions. When used in conjunction with the TurboBooster and/or as an accessory to the Turbo-Tandem System, the devices are indicated for atherectomy of infrainguinal arteries. The 0.014” and 0.018” Over-the-wire (OTW) Turbo-Elite laser catheters are also indicated for use as an accessory to the use of the Turbo-Tandem System in the treatment of femoropopliteal artery in-stent restenosis (ISR) in bare nitinol stents, when used in conjunction with Percutaneous Transluminal Angioplasty (PTA).
Potential adverse events associated with procedures used to treat PAD may include: a sudden, temporary or ongoing re-closure of the treated artery; blood clot or obstruction of the artery by plaque debris; a tear, rupture or damage to the artery (or nearby vein or nerve); minor bleeding or bruising at the entry site. Other complications may occur.
Rare but serious potential adverse events include: the need for urgent additional procedures or surgery due to bleeding, vascular damage, loss of blood flow or other complications; decrease or loss of kidney function due to contrast exposure; the need for amputation due to inability to restore blood flow; and infection, stroke, irregular heartbeat, heart attack or death. This information is not intended to replace a discussion with your healthcare provider on the benefits and risks of this procedure to you.