Device-Type
Intravascular Ultrasound
Manufacturer
Boston Scientific
iLAB™ Ultrasound Imaging System and ULTRA ICE™ PLUS Ultrasound Imaging Catheter for Intracardiac Imaging
ICE provides the combination of real-time imaging and soft tissue visualization that cannot be duplicated by fluoroscopy, pre-operative imaging (CT or MR), or electroanatomic mapping. Not only can you identify anatomical structures, you can visualize where devices are relative to those structures.
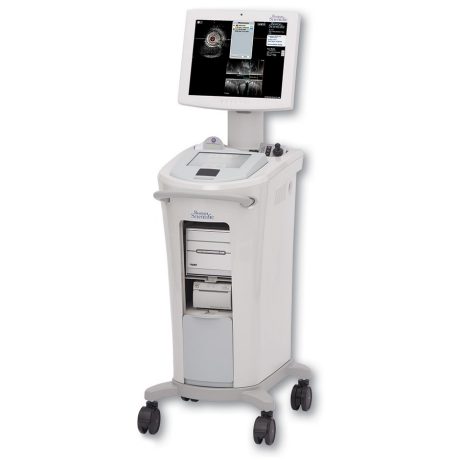
iLAB Ultrasound Imaging System
iLAB transformed visualization during EP procedures as the first intracardiac ultrasound system customized for the EP lab. It offers an easy user interface and automatic enhancement of ICE images. The Modular hardware design comes either installed or on a cart allowing flexibility to upgrade. The iLAB system is compatible with all Boston Scientific ultrasound catheters: Intracardiac (ICE), Intravascular (IVUS) and Peripheral (PI).
ULTRA ICE PLUS Catheter
The Boston Scientific ULTRA ICE PLUS catheter is designed to provide precise, real-time visualization of both intracardiac anatomy and devices positioned within the heart. Not only does it help you in identifying anatomical structures, it also helps you in visualizing where your devices are relative to those structures.
Device Illustration

Unique Vision: 360° Views and Detailed Near-Field Resolution
The ULTRA ICE PLUS Catheter generates a cross-sectional and panoramic 360° image perpendicular to the catheter, with the tip as a central reference point. This allows the user to visualize structures (such as the fossa) directly adjacent to the catheter tip and still see a detailed cross-section of the entire septum.
Image Comparison

Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers