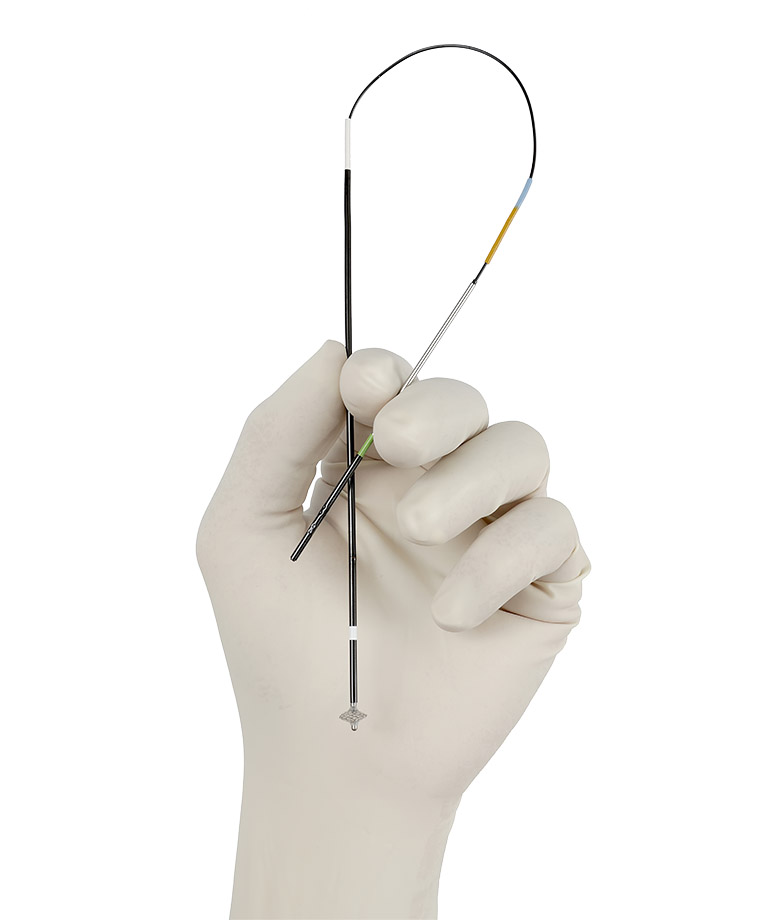
Manufacturer > Cardiva Medical > Devices > VASCADE MVP® VASCULAR CLOSURE FOR PROCEDURES
VASCADE MVP® VASCULAR CLOSURE FOR PROCEDURES
EARLY AMBULATION
A NEW ERA
Introducing VASCADE MVP®
VASCULAR CLOSURE FOR EP1 PROCEDURES
VASCADE MVP eliminates the need for manual compression and reduces time to ambulation by 64%.
Get patients up and moving hours earlier, with significantly less discomfort.
The only FDA-approved closure device for use following cardiac ablations.
Evolution of AF Ablation Workflow

Features and Benefits
Device Documents
Questions & Answers
×