Device-Type
Ablation System
Manufacturer
Medtronic
NONTHERMAL VEIN CLOSURE
The VenaSeal system delivers a small amount of a specially formulated medical adhesive to seal — or close — the diseased vein, rerouting blood to nearby healthy veins, which provides symptom relief.
A MORE COMFORTABLE EXPERIENCE
- Simple outpatient procedure
- No tumescent anesthesia
- Less pain and bruising than thermal ablation
- Faster recovery time than thermal ablation
- Compression stockings not needed after the procedure
The VenaSeal closure system (VenaSeal system) is indicated for use in the permanent closure of lower extremity superficial truncal veins, such as the great saphenous vein (GSV), through endovascular embolization with coaptation. The VenaSeal system is intended for use in adults with clinically symptomatic venous reflux as diagnosed by duplex ultrasound (DUS).
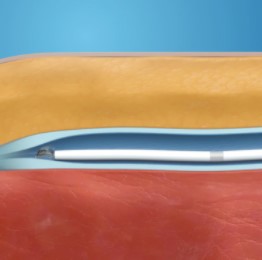
HOW VENASEAL CLOSURE SYSTEM WORKS

Step 1: Adhesive Placed
Adhesive is placed in the vein via small catheter.

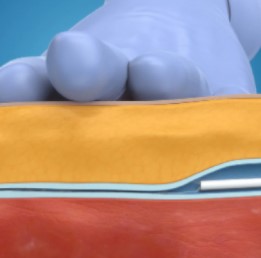
Step 2: Pressure Applied
Pressure is applied to the leg to help seal the vein.

Step 3: Catheter Removed
The small catheter is removed from the vein.

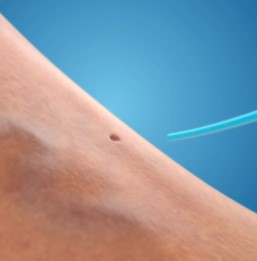
Step 4: Bandage Applied
A bandage is applied to cover the access site.
Safety informations
Potential adverse events
Questions & Answers