Device-Type
Venous Drainage Cannula and Circuits
Targated Speciality
Cardiovascular
Manufacturer
Angiodynamics
The AngioVac System includes the AngioVac Cannula and the AngioVac Circuit. The AngioVac Cannula is intended for use as a venous drainage cannula during extracorporeal bypass for up to six hours and for removal of fresh, soft thrombi or emboli during extracorporeal bypass for up to six hours. Blood that is aspirated with the AngioVac Cannula is simultaneously reinfused back into the patient’s body with the AngioVac Circuit to minimize blood loss.
The AngioVac Circuit is intended for use in procedures requiring extracorporeal circulatory support for periods of up to six hours, and is designed solely for use with the AngioVac Cannula. The AngioVac System is for use with other manufacturer’s off-the-shelf pump, filter, and reinfusion cannula.
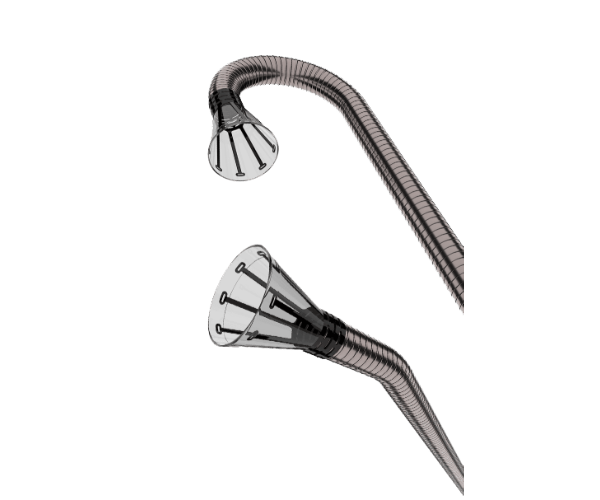
Now in its third generation, the AngioVac System features cannulas with either a 20-degree or a 180-degree angled tip, which facilitate potentially easier navigation and placement through the patient’s venous system. AngioVac’s cannulas utilize radiopaque nitinol tips for visualization under fluoroscopic imaging, with funnel tips that enhance venous drainage flow and prevent clogging of the cannula with commonly encountered undesirable intravascular material such as soft thrombi, emboli, or vegetation.
The AngioVac Cannula is available in a 20-degree and 180-degree angled tip. These options facilitate potentially easier navigation through the patient’s vasculature. The AngioVac Cannula features a self-expanding nitinol funnel shaped tip, which enhances drainage flow when actuated using a sliding over sheath. This sheath aids in funnel capture, deployment, and curve manipulation. The nitinol funnel tip aids in consistent deployment and prevents clogging of the cannula with commonly encountered undesirable intravascular material, such as fresh, soft thrombi, emboli, or vegetation.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers