Manufacturer > Argon Medical Devices > Devices > Atrieve Vascular Snare Kit
Atrieve Vascular Snare Kit
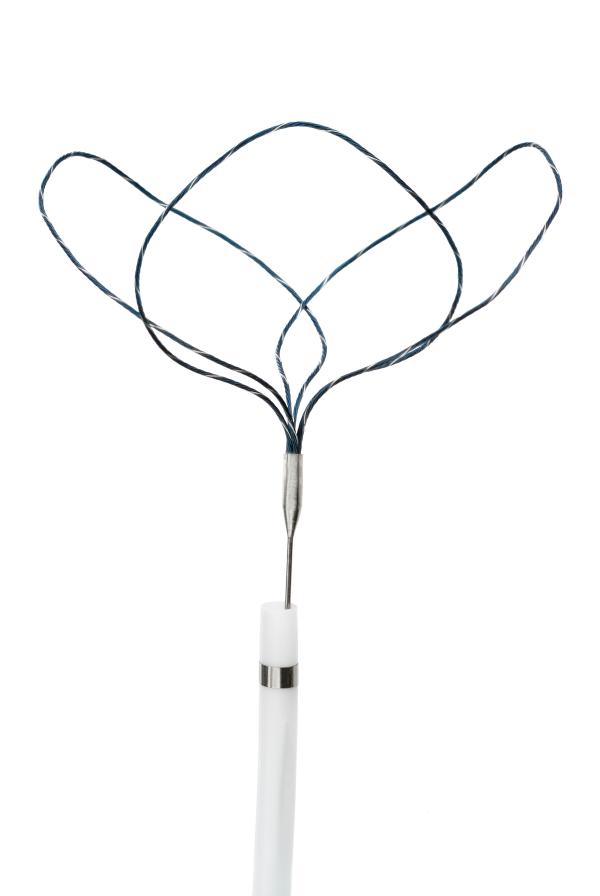
The Atrieve™ Vascular Snare Kit comprises a Snare device with three preformed loops constructed of super-elastic nitinol and platinum. The loops are non-interwoven and can slide relative to one another. The loops of the Snare can be introduced into a delivery catheter without the risk of permanent device deformation. The Atrieve™ Vascular Snare Kit also includes a Delivery Catheter, a torque handle, and an Introducer. The Delivery Catheter has a radiopaque marker band at the distal tip.
Intended Use/Purpose:
The Atrieve™ Vascular Snare Kit is intended for use in the cardiovascular system or hollow viscous to retrieve and manipulate foreign objects.
Indication for Use:
The Atrieve™ Vascular Snare Kit is intended for use in the cardiovascular system or hollow viscous to retrieve and manipulate foreign objects. Manipulation procedures include indwelling venous catheter repositioning, indwelling venous catheter fibrin sheath stripping, and central venous access veni-puncture procedure assistance.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers