Manufacturer > Thrombolex Inc. > Devices > BASHIR™ Plus Endovascular Catheter
BASHIR™ Plus Endovascular Catheter
The BASHIR™ Plus Endovascular Catheter
is intended for the controlled and selective infusion of physician specified fluids, including thrombolytics, into the peripheral vasculature, enabling the restoration of blood flow in patients with venous thrombus.
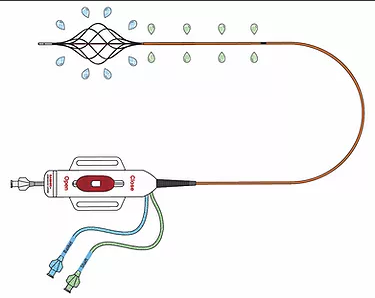
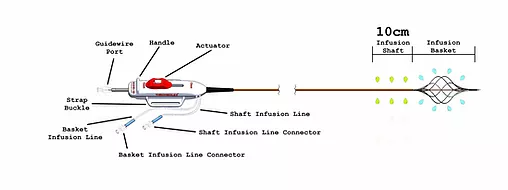
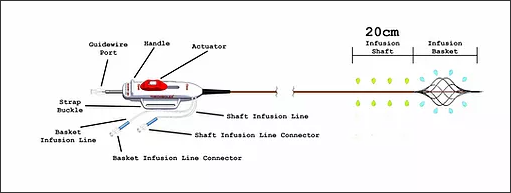
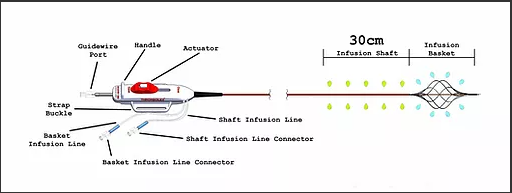
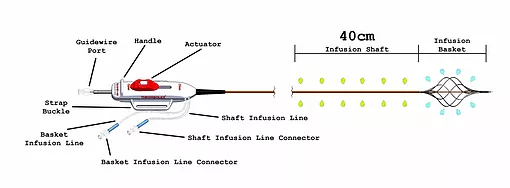
The catheter has a distal infusion segment consisting of an expandable basket with mini-infusion catheters, each with multiple infusion holes (hereafter referred to as “infusion basket”), and a second infusion segment on the catheter shaft, adjacent and proximal to the infusion basket and defined by length of the shaft infusion segment (+10cm,+ 20cm,+ 30cm, or + 40cm) between the proximal end of the infusion basket and a radiopaque marker located proximally on the shaft.
The two infusion segments are independent from each other, each having a dedicated infusion line located on the catheter handle labeled BASKET or SHAFT. The infusion basket can be expanded using the red actuator located on the handle at the proximal end of the device.
After expansion, the mini-infusion catheters may be returned to their original closed positions by depressing the white button on the actuator and advancing the actuator toward the distal end of the device.
The device is used for the delivery of physician-specified fluids at multiple cross-sectional points of the target vessel location and is offered in multiple shaft infusion segment lengths .
The catheter is advanced over a guidewire using standard endovascular interventional techniques and is compatible with standard infusion connectors, accessories and equipment.
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers