Device-Type
Ablation System
Manufacturer
Medtronic
Radiofrequency Ablation for
Chronic Venous Insufficiency (CVI)
The ClosureFast procedure, with its patented overlap design, is the only RFA procedure with published long-term clinical data demonstrating safety and efficacy, with a 91.9% closure rate at 5 years.
The ClosureFast endovenous radiofrequency ablation (RFA) catheter is intended for endovascular coagulation of blood vessels in patients with superficial vein reflux. The catheter is provided sterile, and is a single-use, disposable device. This catheter cannot be adequately cleaned and/or sterilized by the user in order to facilitate safe reuse, and is therefore intended for single use. Attempts to clean or sterilize these devices may result in a bio-incompatibility, infection, or product failure risks to the patient.
SYSTEM COMPONENTS
ClosureFast Endovenous Radiofrequency Ablation Catheter

- The ClosureFast catheter precisely heats a 7 cm vein segment (or 3 cm for shorter, refluxing vein lengths) in one 20-second interval.
- The heat provided by the catheter shrinks and collapses the target vein, creating a fibrotic seal and occluding the vessel.
ClosureFast Endovenous Radiofrequency Ablation Stylet

- The ClosureRFS™ stylet is the only intravascular ablation device specifically intended for the treatment of incompetent perforator and tributary veins.
- This minimally invasive outpatient procedure leaves minimal scarring at the puncture site and can be either the primary treatment or an adjunct treatment using the ClosureFast catheter.
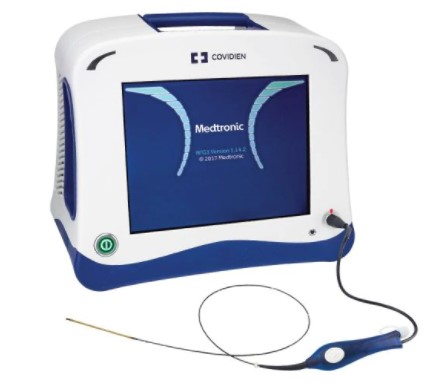
ClosureFast Radiofrequency Generator

- The ClosureRFG generator delivers radiofrequency energy to the ClosureFast catheter and the ClosureRFS stylet for the effective treatment of CVI.
- The controlled feedback mechanism monitors intravascular heat parameters in real time to automatically regulate therapeutic power.
Features and Benefits
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×