Manufacturer > Shape Memory Medical > Devices > IMPEDE-FX Embolization Plug
IMPEDE-FX Embolization Plug
Device-Type
Vascular Plug
Manufacturer
Shape Memory Medical
The IMPEDE-FX Embolization Plug is a shape memory polymer plug. The porous embolic scaffold supports the rapid formation of small interconnected clots throughout its structure. The biocompatible and non-inflammatory polymer promotes rapid conversion to organized thrombus, followed by collagen deposition without chronic active inflammation, which leads to a stable occlusion.
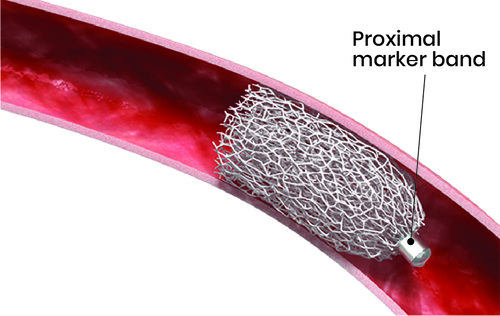
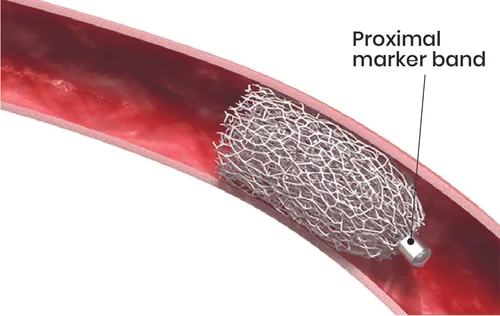
The IMPEDE ®-FX Embolization Plug is a pushable embolization device comprised of a self-expanding porous Shape Memory Polymer (SMP) Plug and a proximal platinum/iridium markerband. The IMPEDE-FX device may only be used in conjunction with the IMPEDE Embolization Plug to further enhance vessel occlusion or increase the length of occlusion within the target vessel.
The device is supplied pre-loaded in an Introducer with the SMP Plug in a crimped state. It is designed to be delivered to the target vessel using a guidewire through a standard catheter/sheath. Upon deployment in the target vessel and exposure to an aqueous environment and body temperature, the SMP Plug will self-expand to embolize the target vessel.
Features and Benefits
Recommended Compatible Devices
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers