Manufacturer > Boston Scientific > Devices > Sentry Bioconvertible IVC Filter
Sentry Bioconvertible IVC Filter
Device-Type
Vena Cava Filter
Manufacturer
Boston Scientific
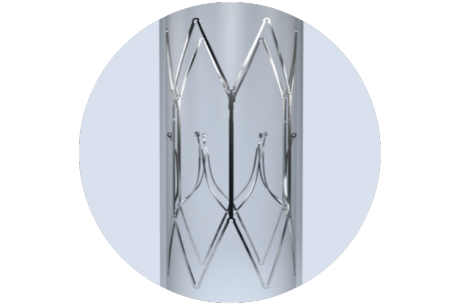
The Sentry Bioconvertible Inferior Vena Cava (IVC) Filter is designed to provide immediate protection against Pulmonary Embolism (PE) in patients at transient risk of PE, without requiring a second retrieval procedure.
One filter. One tool. One procedure.
Sentry is designed to immediately protect against pulmonary embolism (PE) then bioconvert following the period of transient risk, leaving an open, unobstructed lumen. Its unique design gives you control over filter safety and effectiveness and eliminates the need for (and risks of) a second retrieval procedure.
Product Description
Sentry is designed to bioconvert following the transient risk period, to leave a patent, unobstructed lumen, eliminating the need for retrieval procedures, as well as reducing the associated risks and complications of conventional long-term filters.
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers