Device-Type
Mechanical Thrombectomy
Manufacturer
Medtronic
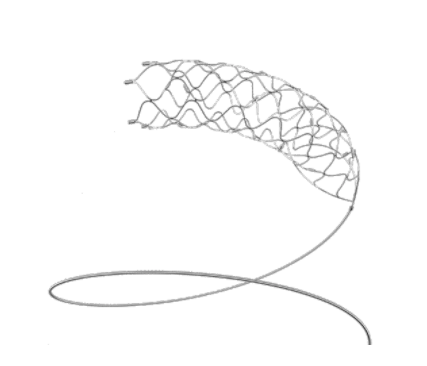
The Solitaire™ X revascularization device, featuring Parametric™ design, a unique overlapping stent retriever-based technology, restores blood flow and retrieves clots from occluded blood vessels in the brain for patients experiencing acute ischemic stroke (AIS) due to a large vessel occlusion (LVO).
The Solitaire™ X portfolio is designed to give you greater confidence during interventional stroke procedures with:
- Unique Parametric design for dynamic clot integration
- Differentiated radial outward force
- Complete visualization and coverage from M2 to ICA
- Optimized delivery system produces lower delivery force for procedural efficiency and smooth navigation
- Robust clinical evidence
Features and Benefits
Use Case Examples
Safety informations
Potential adverse events
Device Documents
Questions & Answers
×