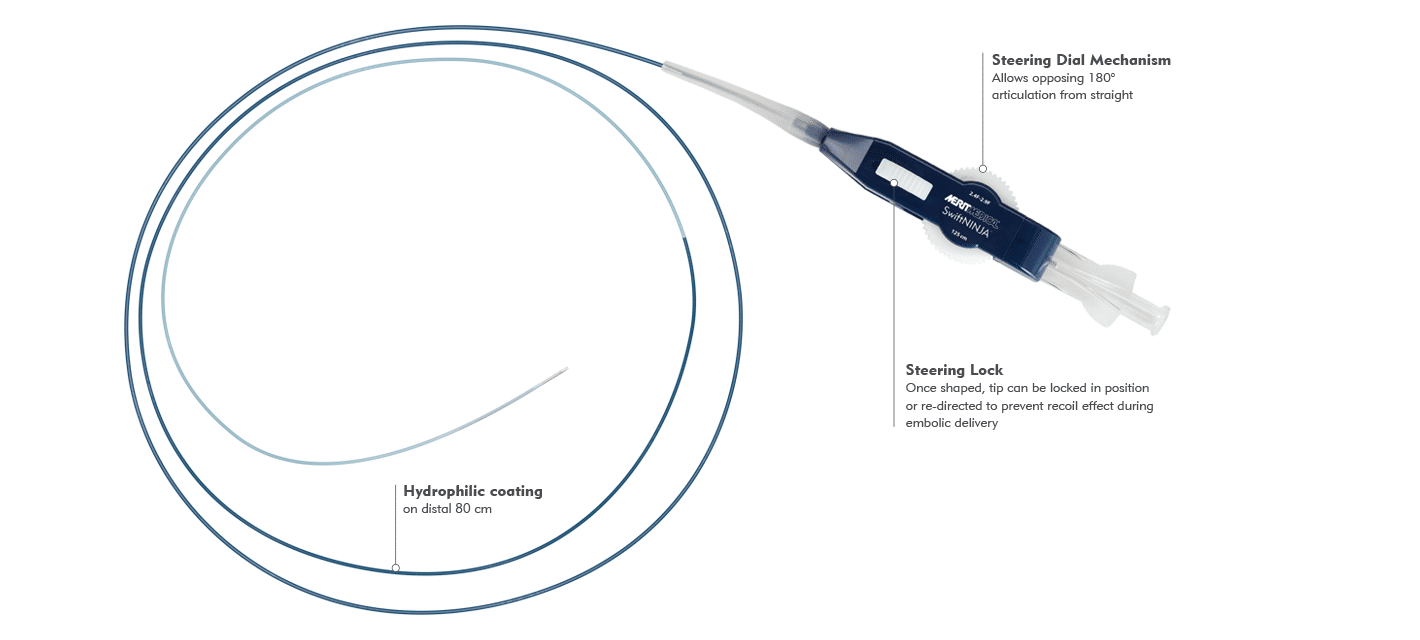
INDICATIONS FOR USE:
The microcatheter is intended for general intravascular use, including peripheral and coronary vasculature. Once the sub selective region has been accessed, the microcatheter can be used for the controlled and selective infusion of diagnostic, embolic, or therapeutic materials into vessels. The microcatheter should not be used in the cerebral vessels.
CONTRAINDICATIONS: None known WARNINGS:
1. This device is intended to be used only by physicians trained in percutaneous intravascular techniques and procedures. Operators should be well trained in microcatheter use and embolization procedures.
2. Advancement of the microcatheter beyond the guide wire may result in vessel trauma.
3. If any abnormality is observed in the microcatheter movement or if the steerable tip is inserted into a location other than the target site, stop the procedure immediately. Identify the position of the steerable tip and the cause of the miss-direction under fluoroscopy. Reposition the microcatheter if necessary. Should the abnormality still remain, stop insertion, relieve any tension on the steerable tip by unlocking the steering dial lock and articulate to a straight tip via the steering dial. Carefully remove all the devices including microcatheter from the vessel to prevent vessel or product damage. Inspect the microcatheter and identify problem. If the microcatheter remains damage free reinsert and resume procedure. Otherwise replace the microcatheter and resume procedure.
4. The infusion dynamic pressure with this microcatheter should not exceed 6,900 kPa/1,000 psi. Infusion pressure in excess of this maximum may result in microcatheter rupture, possibly resulting in patient injury. If flow through the microcatheter becomes restricted, do not attempt to clear the microcatheter lumen by infusion. The static pressure with this microcatheter should not exceed 2,068 kPa/300 psi. Static pressure in excess of this maximum may result in microcatheter rupture, possibly resulting in patient injury. Identify and resolve the cause of the blockage or replace the microcatheter with a new microcatheter before resuming infusion.
5. Avoid passing the microcatheter through a metal lumen such as a stent if possible. If used with metal, use caution during advancement to ensure that the microcatheter does not come in contact with the metal which may damage the coating and/ or decline the lubricity.
6. Do not use a guide wire to help insert embolic material(s). Otherwise, it may cause entrapment of the guide wire between the lumen of microcatheter and the embolic material(s) and lead to failure of embolization.
7. In the event of catheter fracture or separation in the vasculature, stop the procedure immediately. Carefully remove all catheters and devices, including the guiding catheter. Confirm that there is no catheter residue in the vessels under fluoroscopy. In some cases, residue of microcatheter shaft could remain in the stopcock of the guiding catheter because of operation of the stopcock during insertion.
8. In the event that expansion of the microcatheter wall is observed during injection of medication, contrast media, or embolics into the microcatheter by syringe or injector, stop the procedure immediately. Otherwise, the microcatheter may fracture. If any air bubbles are observed in the connector of the microcatheter, remove all air bubbles with a syringe etc. Otherwise, this may lead to risk of air embolism in the vessels. Replace the microcatheter with a new microcatheter prior to proceeding.
9. If any unexpected resistance is felt or if flow through the microcatheter becomes restricted during administration or insertion of embolic materials, medications, or contrast media do not attempt to clear the microcatheter lumen by forced infusion. Identify and resolve the cause of the blockage or replace the microcatheter with a new microcatheter before resuming infusion, otherwise damage to the vessel or product can occur.
10. The static pressure with this microcatheter should not exceed 2,068 kPa/300 psi. Static pressure in excess of this maximum may result in microcatheter rupture, possibly resulting in patient injury. 11. Contents supplied sterile using an ethylene oxide (ETO) process.
12. Sterile if package is unopened and undamaged.
13. For single patient use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing or resterilization may compromise the structural integrity of the device and/or lead to device failure which, in turn, may result in patient injury, illness or death. Reuse, reprocessing or resterilization may also create a risk of contamination of the device and/or cause patient infection or cross-infection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness or death of the patient.
14. After use, dispose of product and packaging in accordance with hospital, administrative, and/or local government policy.
15. Make sure that the guiding catheter does not slip out of the vessel. If the guiding catheter should leave the vessel when the microcatheter and/or the guide wire is moved, this may result in the kinking and/or damage of the microcatheter system.
16. Do not use a power injector to infuse agents other than contrast media, as the microcatheter may become blocked. The safety setting of injection pressure must not exceed the maximum dynamic injection pressure of 6,900 kPa/1,000 psi. Exceeding injection pressure beyond the maximum dynamic injection pressure may cause microcatheter rupture. (See Instructions for Using a Power Injector)
17. Do not immerse, apply, or wipe microcatheter in any medication containing an organic solvent such as alcohol for disinfection. Otherwise, it may cause damage to product and/or loss of lubricity.
18. If a guiding catheter with a stopcock is used, do not operate the stopcock during insertion of microcatheter, otherwise, damage or fracture may occur.
19. Do not attempt to manually pre shape the microcatheter tip by heat, a metal shaping tool, pinching, bending or crushing, otherwise, breakage of steerable tip and/or decline in articulation may occur.
20. Do not operate the steerable tip while a guide wire is positioned distal to the microcatheter tip, otherwise, vascular damage and/ or breakage of wire tip or microcatheter tip may occur.
PRECAUTIONS:
1. : Federal law (U.S.A.) restricts this device to sale by or on the order of a physician.
2. Ensure embolic material compatibility with microcatheter prior to use.
3. Always monitor infusion rates when using the microcatheter.
4. When injecting contrast for angiography, ensure that the microcatheter is not kinked, occluded, or directed toward the vessel wall.
5. The microcatheter has a lubricious hydrophilic coating on the outside of the microcatheter. It must be kept hydrated prior to removal from its carrier and during the actual procedure in order to be lubricious. This can be accomplished by attaching the Y-connector to a continuous saline drip.
6. Prior to a procedure, all equipment to be used for the procedure should be carefully examined to verify proper function and integrity.
7. Inspect the microcatheter prior to use for any bends or kinks. Any microcatheter damage may decrease the desired performance characteristics.
8. Exercise care in handling of the microcatheter during a procedure to reduce the possibility of accidental breakage, bending or kinking.
9. When the microcatheter is in the body, it should be manipulated only under fluoroscopy. Do not attempt to move the microcatheter without observing the resultant tip response.
10. Never advance or withdraw an intravascular device against resistance until the cause of the resistance is determined by fluoroscopy. Movement of the microcatheter or guide wire against resistance may result in separation of the microcatheter or guide wire tip, damage to the microcatheter, or vessel perforation. Because the microcatheter may be advanced into narrow sub selective vasculature, repeatedly assure that the microcatheter has not been advanced so far as to interfere with its removal.
11. Excessive tightening of a hemostatic valve onto the microcatheter shaft may result in damage to the microcatheter.
12. Read and follow the manufacturer’s IFU for diagnostic, embolic, or therapeutic agents to be used with this microcatheter.
13. Do not use opened or damaged packages.
14. Use prior to the “use before” date.
15. Store at controlled room temperature.
16. Before injecting medication and/or contrast media into the microcatheter by syringe or injector, make sure that the two connectors are securely attached to each other. If using a mechanical injector utilize a connection tubing rated to the maximum pressure rating 6,900 kPa/1,000 psi otherwise, it may cause leakage of medication and/or contrast media, and/or damage to the syringe, injector, and/or microcatheter.
17. Do not administer or insert embolic materials, medication, and contrast media etc. with pressure, or insert a guide wire into microcatheter if the microcatheter shaft is kinked or twisted. Otherwise, it may cause damage to microcatheter.
18. Exchange microcatheters frequently during lengthy procedures that require extensive manipulation or multiple guide wire exchanges.
19. Do not rotate the microcatheter more than 5 full rotations in the straight position if the tip does not rotate as microcatheter damage may occur.